Spironolactone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
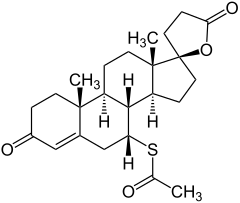
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Spironolactone | ||||||||||||||||||
| other names |
7 α -acetylthio-3-oxo-17 α -pregn-4-ene- 21,17 β- carbolactone |
||||||||||||||||||
| Molecular formula | C 24 H 32 O 4 S | ||||||||||||||||||
| Brief description |
white powdery solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 416.57 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
207-208 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Spironolactone acts as a competitive antagonist on the mineralocorticoid receptor and is assigned to the group of potassium-sparing diuretics . It is used to reduce the effect of aldosterone when there is an increased release of this endogenous hormone and to treat heart failure . Due to the reduced effect of aldosterone, more sodium is excreted and potassium is retained, since the sodium channel installation caused by aldosterone does not occur. As a result, there is an increased excretion of water .
Spironolactone was approved and registered for therapy in Germany in October 1967. It is on the WHO Essential Medicines List .
effect
In the collecting ducts of the kidneys, spironolactone blocks the binding of the mineral corticoid aldosterone to its receptor. This prevents the incorporation of epithelial sodium channels (ENaC) into the luminal membrane of the main cells and thus effectively inhibits sodium reabsorption. Due to the lack of sodium reabsorption, water is not drawn into the collecting tube cells and thus into the blood - more water and sodium are excreted. This reduces the amount of blood: the heart is relieved and the blood pressure drops. Accumulations of water in the tissue ( edema ) are also flushed out. The loss of potassium due to other diuretics (e.g. thiazides) can be compensated by a combination treatment with spironolactone, since the lack of ENaC channels means that there is no compensatory, charge-balancing loss of potassium via luminal ROMK channels. Actually, for every sodium ion taken up into the collecting tube cell via the ENaC channel, a potassium ion would be forced out of the cells via ROMK channels into the collecting tube lumen. This is prevented secondarily by spironolactone, which is why hyperkalemia can arise as a significant side effect.
Clinical studies have shown that the administration of spironolactone has a beneficial effect on the course of the disease and survival in chronic heart failure, regardless of its antihypertensive effect.
Pharmacokinetics
- Absorption: After oral administration, spironolactone is well absorbed and eliminated from the plasma within one hour. However, various metabolic products ( metabolites ) of the starting substance remain. It is assumed that it is not spironolactone but mainly its metabolites that cause the pharmacological effect. The metabolism of spironolactone is complex and takes place in different ways. An important active metabolite is canrenone , which appears in the blood and urine, but is still further metabolized. Another important metabolite is 7 α thiomethyl-spironolactone, which is likely to be produced in the kidney and liver. In addition to the urine, many metabolites are also excreted in the bile and stool .
- Bioavailability: After oral administration, more than 90% is available in humans.
- Onset of action: The effect occurs slowly. It takes about two to three days for a clinical effect to be seen. The maximum diuretic effect occurs after five days. Even increasing the dose cannot shorten the time it takes to take effect.
indication
Spironolactone is used for therapy in the case of increased aldosterone levels. These can be increased production, e.g. B. in primary aldosteronism , a reduced breakdown, z. B. in the context of liver cirrhosis (secondary hyperaldosteronism), or a pseudohyperaldosteronism are the basis. In addition, spironolactone is approved for the treatment of chronic heart failure. Antiandrogenic therapy with spironolactone is rare in Europe and standard in the USA, since CPA ( cyproterone acetate ) is not approved by the FDA. It takes place in doses of 100–200 mg. This substance has the advantage that the well-known side effect of breast growth is desirable and high blood pressure (hypertension) can be treated at the same time.
The use of spironolactone is not indicated in the presence of hyperkalaemia , pronounced renal insufficiency or in Addison's disease .
Side effects
The most common side effect is an increase in blood potassium levels , especially when ACE inhibitors and AT1 antagonists are given at the same time . As a result, the use of the drug requires regular control of the serum potassium over the duration of the application. Temporary allergic skin reactions occur in around 2% of patients. Due to the structural relationship between spironolactone and steroid hormones, hormonal disorders can occur at high dosages. In women, long-term use can lead to masculinization of the hair type and the absence of menstrual bleeding, in men gynecomastia and erectile dysfunction can occur. Spironolactone can also cause potentially irreversible hoarseness.
These side effects are explained by the unselective effect of spironolactone, which, especially from doses of over 100 mg, also has effects on the other steroid receptors (androgens and glucocorticoids). As another representative of the aldosterone antagonists, eplerenone acts selectively on the mineral corticoid receptor.
Interactions
In combination with digoxin , increased plasma levels of cardiac glycoside occur. Additional intake of acetylsalicylic acid can inhibit the diuretic effect of spironolactone. In combination with lithium , the Li level in the blood is increased and drug poisoning can occur if the amount of Li consumed is not reduced.
Abuse in Sports
In body building , spironolactone is a popular doping agent to lower the aldosterone level before competitions and thus excrete subcutaneous water (water that is usually deposited under the skin through testosterone administration).
Female users also use it to lower the androgen level, which is increased by the intake of artificial male hormones and which leads to "masculinization" in women. With an overdose and prolonged use, the risk of kidney damage increases enormously.
Trade names
Aldactone (D, A, CH), Jenaspiron (D), Osyrol (D), Spirorben (A), Verospiron (D), Xenalon (CH), numerous generics (D, A)
Aldactone Saltucin (A), Aldozone (CH), Furorese comp. (D), Furospir (CH), Furospirobe (A), Lasilacton (A, CH), Osyrol-Lasix (D), Sali-Aldopur (A), Spiro-comp. (D, A), Spiro D-Tablinen (D), Spironothiazide (D)
Individual evidence
- ↑ a b c d e f Spironolactone data sheet from Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ↑ a b Return of an old friend? Spironolactone. The RALES study. - Medicines letter.
- ↑ B. Pitt, F. Zannad, WJ Remme, R. Cody, A. Castaigne, A. Perez, J. Palensky, J. Wittes: The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. In: N Engl J Med . 2; 341 (10) (1999), pp. 709-717. PMID 10471456 .
- ↑ a b c J. Greven, HJ Kramer: Therapy of edema. In: Björn Lemmer , Kay Brune (Eds.): Pharmacotherapy - Clinical Pharmacology. 13th edition. Heidelberg, 2007, pp. 60-62.
- ↑ Dickstein et al: ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. ( Memento from January 12, 2012 in the Internet Archive ) In: European Heart Journal . 29 (2008), pp. 2388-2442.
- ↑ Film Coated Tablet Dosage Form . In: Definitions . Qeios, February 7, 2020, doi : 10.32388 / o3qyj9 .
- ↑ a b Spironolactone - Flexicon (DocCheck)
- ↑ a b James M Ritter, Lionel D Lewis, Timothy GK Mant, Albert Ferro: Clinical Pharmacology and Therapeutics . 5th edition. London 2008, p. 276, p. 76f.
- ↑ Michael Freissmuth, Stefan Böhm: Pharmacology and toxicology: From the molecular basis for pharmacotherapy . Springer-Verlag, 2012, ISBN 978-3-642-12354-2 ( google.de [accessed on March 6, 2019]).
- ↑ Yellow list
- ↑ Justin B. Usery, Timothy H. Self: Lithium-Drug Interactions , 2008-01-01.
- ^ WebMD: Interactions: ACE Inhibitors; ARBs / Lithium , downloaded March 22, 2017.
- ↑ The Black Book - Anabolic Steroids. 2007, ISBN 978-3-00-020944-4 , pp. 456/457.