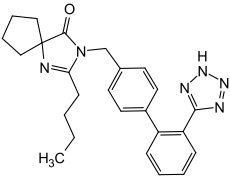
Irbesartan
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| Structural formulas of the two tautomeric forms: polymorph A (top) and polymorph B (bottom) | |||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Irbesartan | ||||||||||||||||||
| other names |
2-butyl-3 - ({4- [2-1 H -1,2,3,4-tetrazol-5-yl) phenyl] phenyl} methyl) -1,3-diazaspiro [4.4] non-1-en -4-on ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 25 H 28 N 6 O | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 428.54 g · mol -1 | ||||||||||||||||||
| Melting point | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Irbesartan is a drug from the AT 1 antagonist group that is used in the treatment of high blood pressure and mild to moderate heart failure when ACE inhibitor therapy is unsuitable.
Application areas (indications)
Irbesartan is used to treat the following diseases:
- Essential hypertension
- Kidney disease in patients with hypertension and type 2 diabetes mellitus as part of antihypertensive treatment.
Presentation and extraction
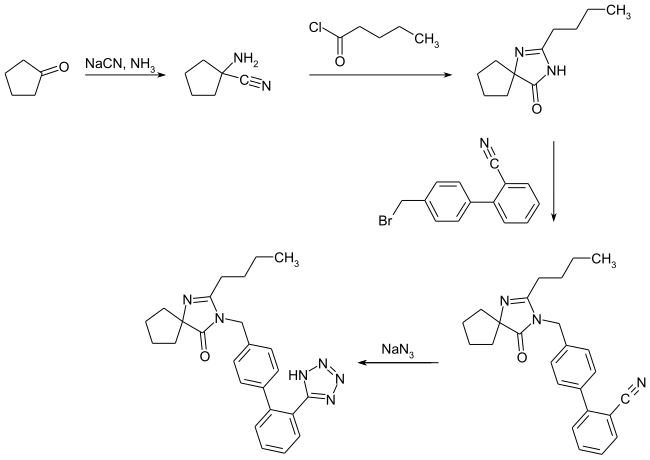
The multi-stage synthesis of irbesartan starts from cyclopentanone , the 1-amino-1-cyclopentanecarbonitrile being obtained in the first step by reaction with sodium cyanide and ammonia . A spiro intermediate compound is formed via amide formation with pentanoic acid chloride and subsequent basic cyclization . Then in the presence of sodium hydride and dimethylformamide with 4 '- (bromomethyl) -biphenyl-2-carbonitrile the biphenyl substructure is inserted into the molecule . In the last synthesis step, the target compound is obtained by means of sodium azide by forming the tetrazole function from the cyano group .
properties
The compound occurs in two polymorphic crystal forms. Polymorph A melts at 181 ° C with a heat of fusion of 91 J g −1 , polymorph B at 186 ° C with 116 J g −1 . Both forms are monotropic to one another. The polymorph B is the thermodynamically stable crystal form. The two polymorphic crystal forms differ with regard to the tautomerism of the tetrazole structure. Polymorph A corresponds to the 4 H tetrazole structure, polymorph B to the 2 H tetrazole structure. Polymorph A crystallizes in a hexagonal crystal system , while polymorph B in a triclinic crystal system .
Carcinogenic contaminants
Due to contamination with the potentially carcinogenic N -nitrosodiethylamine (NDEA) at the active ingredient manufacturer Aurobindo Pharma Limited from Hyderabad, there have been several recalls of drugs containing Irbesartan since October 2018.
Individual evidence
- ↑ a b c d e N. Boutonnet-Fagegaltier, J. Menegotto, A. Lamure, H. Duplaa, A. Caron, C. Lacabanne, M. Bauer: Molecular Mobility Study of Amorphous And Crystalline Phases of a Pharmaceutical Product by Thermally Stimulated Current Spectrometry. In: J Pharm Sci . Volume 91, 2002, pp. 1548-1560, doi: 10.1002 / jps.10146 .
- ↑ a b data sheet Irbesartan, European Pharmacopoeia (EP) Reference Standard from Sigma-Aldrich , accessed on November 1, 2016 ( PDF ).
- ↑ RED LIST 2013. 53rd edition. Verlag Rote Liste Service, Frankfurt am Main, ISBN 978-3-939192-70-1 , p. 662.
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher, Dieter Reichert: Pharmaceutical Substances. 4th edition. 2 volumes. Thieme-Verlag, Stuttgart 2000, ISBN 1-58890-031-2 ; online since 2003 with biannual additions and updates.
- ↑ M. Bauer, RK Harris, RC Rao, DC Apperley, CA Rodger: NMR study of desmotropy in irbesartan, a tetrazole-containing pharmaceutical compound. In: J. Chem. Soc. Perkin Trans. 1998, pp. 475-481.
- ↑ Z. Böcskei, K. Simon, RC Rao, A. Caron, CA Rodger, M. Bauer: Irbesartan crystal form B. In: Acta Cryst. C 54, 1998, pp. 808-810.
- ↑ NDEA: Second Irbesartan recall, first irbesartan CEP withdrawn . In: Deutsche Apotheker Zeitung . October 11, 2018.