Valsartan scandal
The valsartan scandal (also sartan scandal ) comprising the occurrence of incidents probably carcinogenic impurities in hypertensive agents from the active substance group of sartans .
Contamination of the active ingredient valsartan from a Chinese manufacturer became known for the first time in July 2018 , followed by cases of valsartan from other manufacturers and other sartans.
Detection and initial drug recalls
The Federal Institute for Drugs and Medical Devices (BfArM) announced on July 4, 2018 about an EU-wide batch-related recall of drugs containing valsartan, the active ingredient of which had been produced by the Chinese manufacturer Zhejiang Huahai Pharmaceutical (ZHP). The trigger for the recalls was a production-related contamination of the active ingredient with N- nitrosodimethylamine (NDMA) ; this substance is classified by the International Agency for Research on Cancer (IARC) and the EU as probably carcinogenic in humans . Patients who take valsartan-containing medicines should not stop taking the medicine without consulting their doctor, as the health risk of discontinuation is many times higher than the possible risk of contamination. There is no acute patient risk. The BfArM explained that it had been informed by a state surveillance authority. The report is based on the test results of the active ingredient manufacturer, which were carried out as part of the production for a finished drug manufacturer from Spain. The BfArM gave neither the name of the reporting state surveillance authority nor the time of its information. According to the former head of toxicology at the Berlin Charité, Ralf Stahlmann, the contamination was not discovered during prescribed controls, but only after an anonymous tip.

On July 5, 2018, the European Medicines Agency (EMA) published, after the discovery of an contamination with NDMA, upon request by the European Commission in accordance with Article 31 of Directive 2001/83 / EC , it was reviewing drugs containing valsartan from the manufacturer Zhejiang Huahai contained. As a precautionary measure, national authorities in the EU have already recalled some drugs containing the active ingredient. The EMA stated in both publications that the contamination with NDMA was "unexpected". On the other hand, others take the position that both the active ingredient manufacturer and the auditors of the European Medicines Quality Authority (EDQM) should have noticed the risk of the NDMA formation as early as 2012. In that year, the EDQM approved the suitability of the test method used by the manufacturer for valsartan. For this purpose, the company Zhejiang Huahai dutifully submitted a change to the synthesis route to the EDQM at the time, which clearly posed the risk of NDMA formation. The contamination with NDMA was "foreseeable" due to the known reactivities of the reagents used.
The fact that the EDQM experts also had no suspicions despite the documents presented to them indicates gaps in the test procedure. According to the chairman of the Drugs Commission of the German Medical Association , the events are “worrying and show that existing measures for quality control and monitoring drug safety sometimes do not work. [...] In particular, the state authorities responsible for drug monitoring act too slowly, are obviously often overwhelmed with these problems and usually do not have sufficiently competent staff to adequately perform this responsible task. "
Toxicological background
NDMA ( N -nitrosodimethylamine ) is a particularly dangerous representative of nitrosamines . The BfArM initially presented the spectrum of possible damage caused by NDMA incompletely by only pointing out that NDMA has been classified by the IARC and the EU as “probably carcinogenic in humans”. In fact, however, NDMA itself and above all its strongly methylating degradation products are also considered to be genotoxic . In addition, there are no known animal species in which NDMA does not cause cancer, and even the smallest amounts of NDMA can have a carcinogenic effect there, which makes it difficult to establish a safe toxicological threshold. In animal experiments with rats in which the diet was mixed with 2000 µg NDMA per kg food over a period of 120 weeks, one of 37 test animals developed a liver tumor; 10 of 12 animals with 50,000 µg per kg food became ill. Due to the unknown threshold, there is no ADI ( Acceptable Daily Intake ) limit for a daily allowable amount of NDMA. Official statements such as “only slightly increased concentration” are therefore not appropriate. As for other genotoxic carcinogens, it must be required to minimize exposure as much as possible and to apply the ALARA principle (“As Low as Reasonably Achievable”).
In the Federal Republic of Germany, it is true that the monitoring of drug traffic and the monitoring of good manufacturing practice (GMP) are the responsibility of the federal states. But apart from a supervisory authority in Bavaria, no state or federal authority has published its own measured values for the contamination of valsartan with NDMA. Instead, both the BfArM and the other state authorities refer to measurements that the Central Laboratory of German Pharmacists (ZL), an association under private law, only carried out on its own initiative at the end of July 2018.
Chemical background
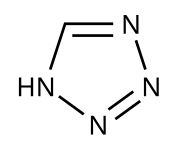
For the development of carcinogenic nitrosamine compounds such as N -nitrosodimethylamine (NDMA), synthetic steps to build up the tetrazole ring found in the molecule of certain sartans are considered to be the cause.
It is reported that Zhejiang Huahai Pharmaceutical (ZHP) published a patent for an improved synthesis of valsartan in 2014. While in the earlier synthesis variant the tetrazole ring was built up from a cyano-substituted , aromatic intermediate stage by 1,3-dipolar cycloaddition using tributyltin azide , in the new synthesis the cyclization reaction takes place using sodium azide , under catalysis with zinc chloride and in the presence of sodium nitrite in an acidic medium . In this synthesis step, dimethylformamide (DMF) acts as a solvent. Under unfavorable reaction conditions during the synthesis process, the degradation product dimethylamine (DMA) can form in DMF and when DMA meets nitrite ions, NDMA can be formed. This contamination can not be determined with the test method in the currently valid pharmacopoeia , since the monograph based on other synthesis methods is geared towards the presence of other impurities and not the determination of NDMA.
The advantage of the Valsartan synthesis route modified by ZHP is said to be that handling the highly toxic and explosive tributyltin azide is dispensed with in favor of reagents that are easier to use. Aspects of the purification of the active ingredient and the synthesis yield can also play a role.
In addition to NDMA, the formation of a number of other volatile and non-volatile nitrosamines in sartan syntheses is also conceivable. Depending on which amine comes together with which nitrosylating reagent under which conditions, different nitrosamines could be formed. The solvent used also plays an important role. However, the ZL estimates the probability that the essential nitrosamines were recorded with NDMA and N-nitrosodiethylamine (NDEA), based on the current data, as high.
In the case of solvents used by other active ingredient manufacturers such as toluene or o -Xylene instead of DMF, a reaction with sodium nitrite to form NDMA is excluded.
More sartan cases
- Valsartan
In addition to the Chinese manufacturer Zhejiang Huahai, contamination by the cancer-endangering NDMA has been detected in batches of valsartan from other manufacturers: The US FDA announced on August 9, 2018 that Valsartan had also been contaminated with NDMA from production by the Indian active ingredient manufacturer Hetero Labs Limited and therefore drugs containing valsartan from this source would be recalled from the US market. According to the BfArM, drugs on the German market were not affected. On August 10, 2018, it was informed at European level that slightly increased NDMA values were also measured at the Chinese manufacturer Zhejiang Tianyu . The government of Upper Bavaria therefore ordered the recall of the only batch affected in Germany on August 14, 2018.
- Irbesartan
Numerous recalls of preparations containing the tetrazole sartan irbesartan have been reported to the German Pharmacists' Drug Commission (AMK). An irbesartan preparation was withdrawn from the market for the first time in July 2018. No contamination could be determined analytically. In October 2018, the EDQM withdrew the certificate of conformity (CEP) for irbesartan from the Indian active ingredient manufacturer Aurobindo Pharma after small amounts of NDEA were detected. In January 2019, Zhejiang Huahai Pharmaceutical lost its CEP.
- Losartan
The active ingredient losartan was also affected by nitrosamine contamination: in September 2018, traces of the potentially carcinogenic substance N-nitrosodiethylamine (NDEA) were found in losartan from the Indian manufacturer Hetero Labs. At the end of December 2018, two contaminated drug batches were recalled in Germany. In January 2019, Zhejiang Huahai Pharmaceutical's certificate of conformity (CEP) for losartan was withdrawn by the EDQM . In February 2019, there were recalls due to the first time found nitrosamine compound N -Nitroso- N -methyl-4-aminobutyric acid (NMBA).
- Candesartan
Almost a year after the first discovery of nitrosamines in sartans , the occurrence of the nitrosamine N-nitrosodiethylamine (NDEA) in very small amounts was described for the first time in June 2019 for candesartan , the fourth sartan with a tetrazole ring.
Consequences
The contamination of a drug with a chemical that is considered to be carcinogenic in humans, presumably over a period of six years, affects a very large number of people: in 2017, according to the local Federal Ministry of Health, around nine million packs of drugs containing valsartan were prescribed in Germany alone . Since around 40 percent of the batches are contaminated, around 900,000 patients could be affected.
Risk assessment
NDMA belongs to the group of nitrosamines. Normally, a Western European consumes an average of 0.3 µg nitrosamines per day with their food. When smoking 20 cigarettes a day, the amount consumed can increase to 17 to 85 µg. Measurements by authorities and organizations such as the Central Laboratory of German Pharmacists have shown between 3.7 and 22 µg NDMA per tablet in the recalled drugs. A single valsartan tablet could therefore contain 75 times the amount of NDMA than the total amount of nitrosamines normally consumed from food per day. The maximum permissible value for NDMA in drinking water is 0.1 µg / l , for beer the technical guideline value of 0.5 µg nitrosamines per kg is regularly undercut. One kg of smoked ham produced using today's method contains around 2.5 µg of nitrosamines, just a tenth of what was found in some tablets.
The EMA repeatedly stated that there was “no acute health risk” from contamination of valsartan drugs. The same agency publishes, however, that for every 5,000 patients who have taken the affected drugs daily with the highest dose of valsartan (320 mg) over a period of seven years, there is an estimated one additional cancer case. Similarly, assuming that patients have also taken NDMA-contaminated valsartan at the highest dose (320 mg) for four years, the FDA estimated that an additional case of cancer could occur in 8,000 such patients.
The estimated additional cases of cancer caused by NDMA in valsartan drugs must be seen against the background that around one in three Europeans will develop cancer at some point in their life.
Claims related to the valsartan affair
As a consequence of the valsartan process, specialists in toxicology and drug quality derive the following requirements:
- Stricter monitoring of active ingredient manufacturers, especially when manufacturing processes change
- Predictive screening analysis on the part of the finished drug manufacturer in order to identify changes in the manufacturing process that have not been notified
- more material, human and technical resources and better mutual coordination between the responsible authorities
- Ending the dependence of industrialized countries on pharmaceutical products from low-wage countries
- more transparency in the pharmaceutical market
Analogous to the long-term effects of cancer-endangering substances in the world of work, for example, according to the weekly newspaper “ Die Zeit ”, questions such as: Do we have to monitor the health of the affected patients? Who is legally responsible for any damage?
After the discovery of further impurities valeramide (VLA) and dimethylvaleramide (VLA-DIM) , which have not been described so far - which are not nitrosamines considered to be carcinogenic - scientists again called for drug manufacturing processes to generally be subjected to a broader review, since it It is unlikely that only sartans are affected.
Measures according to CHMP opinion
On July 5, 2018, the European Commission initiated a risk assessment process for valsartan, which was expanded to include medicines containing candesartan, irbesartan, losartan or olmesartan in September 2018 . Sartans without a tetrazole ring such as azilsartan , eprosartan and telmisartan were not included in the review. The evaluation was carried out by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA), which published its opinion in February 2019. As a result, for the majority of sartan-containing medicinal products, the contaminants NDMA and NDEA were either undetectable or only present in very small amounts. According to new calculations, the EMA estimates the increase in the risk of developing cancer at some point in life compared to the basic risk as very low. According to the CHMP, pharmaceutical companies should in future take all necessary measures to ensure that such impurities do not occur. In the long term, no more nitrosamine impurities should be measurable, i. H. the quantities must be below the detection limit of 0.03 ppm . A transition period of two years should enable companies to make the necessary changes in their manufacturing processes and to introduce test procedures that are sensitive and specific enough to detect the smallest amounts of these impurities in the active ingredients produced. Until then, the CHMP proposes the temporary use of limit values based on a maximum permissible daily dose of 96.0 ng NDMA or 26.5 ng NDEA and derived from animal studies.
With the decision of the EU Commission in April 2019, the CHPM report became legally binding for all EU member states; In March 2020, the authorities in Germany ordered the suspension of drug approvals for which the planned risk-minimizing measures had not yet been implemented or not fully implemented.
The pharmacopoeia monographs of the affected sartans were updated with the 10th edition of the European Pharmacopoeia (Ph. Eur. 10) from July 1, 2019 and limit values for the nitrosamines NDMA and NDEA were added.
The occurrence of nitrosamine-containing impurities in drugs, including other substances such as N -nitrosoethylisopropylamine (EIPNA), N -nitrosodiisopropylamine (DIPNA) and N -nitroso- N -methyl-4-aminobutyric acid (NMBA), was the subject of further investigations by the EMA and national drug authorities . In August 2019, the EMA supplemented its February paper with transitional limit values for DIPNA, EIPNA and NMBA. As for NDMA and NDEA, permissible daily doses of a maximum of 96.0 ng (for NMBA) and 26.5 ng (DIPNA, EIPN) were used.
The US agency US-FDA also specifies limit values for nitrosamines in sartans.
Other drug classes
Sensitized by the sartan incidents, the authorities have expanded their investigations to include other drugs.
In April 2019, the European Medicines Agency (EMA) reported findings of small amounts of NDMA in some batches of the anti-diabetic drug pioglitazone from an Indian manufacturer. In September 2019, both the Food and Drug Administration (US-FDA) and the EMA announced the occurrence of NDMA in some drug batches that contain the gastro-protective agent ranitidine . The EMA wants to assess whether there is a potential risk to patients who have taken contaminated ranitidine preparations for a long time and has initiated a risk assessment process; in the USA the US FDA therefore withdrew all prescription and non-prescription ranitidine medicines from the market in April 2020 . In December 2019, some metformin drugs contaminated with NDMA were discovered in Asia . Metformin is used to treat type 2 diabetes .
Guidelines to avoid nitrosamine contamination
The EMA's Committee on Medicinal Products for Human Use (CHMP) will develop guidelines on how to avoid nitrosamine contamination in medicinal products for human use that contain chemically synthesized active substances.
Web links
- Nitrosamine impurities in medicines , on the European Medicines Agency website
Individual evidence
- ↑ Federal Institute for Drugs and Medical Devices: Valsartan: batch-related recall of drugs containing valsartan, the active ingredient of which was produced by the Chinese manufacturer Zhejiang Huahai Pharmaceutical , press release number 5/18 of July 4, 2018, accessed August 29, 2018.
- ↑ a b Rapid Alert of the BfArM on valsartan , as of August 24, 2018, accessed on August 29, 2018.
- ↑ a b BfArM: Background to the Rapid Alert on Valsartan , without date, accessed August 29, 2018.
- ↑ a b Julia Borsch: ZL detects NDMA in valsartan samples. In: ptaheute .de , July 25, 2018, accessed August 31, 2018.
- ↑ EMA reviewing medicines containing valsartan from Zhejiang Huahai following detection of an impurity. Some valsartan medicines being recalled across the EU , online July 5, 2018, accessed August 29, 2018, pdf (199 kB) ema.europa.eu
- ↑ Update on review of valsartan medicines following detection of impurity in active substance - Assessing potential impact on patients is priority , online July 17, 2018, accessed August 29, 2018.
- ↑ a b c Jakob Simmank : Valsartan: Blood pressure tablets under serious suspicion of cancer , in: Die ZEIT, online July 27, 2018, accessed August 29, 2018.
- ↑ D. Uhl: The control failure in the valsartan affair , Deutsche Apotheker Zeitung, August 2, 2018.
- ↑ a b c Dustin Grunert: Drug safety: The valsartan case shows the industry's Achilles heel , Ärzteblatt, online August 20, 2018, accessed August 29, 2018, pdf aerzteblatt.de .
- ↑ Volume 17 (1978) Some N-Nitroso Compounds , IARC Monographs on the Evaluation of Carcinogenic Risks to Humans.
- ↑ a b c Mona Abdel-Tawab u. a .: Valsartan: ZL finds NDMA in tablets , Pharmazeutische Zeitung, issue 30/2018, accessed August 29, 2018.
- ↑ H. Buschmann, U. Holzgrabe: NDMA in Valsartan - A Search for Traces , Deutsche Apotheker Zeitung 2018, No. 29 of July 19, 2018, p. 22 ff.
- ↑ a b c Sartan production - risk factor ring synthesis , interview with Professor Dr. Mona Tawab (ZL), Pharmazeutische Zeitung, November 9, 2018.
- ↑ D. Cicek-Görkem, N. Tröbitscher, P. Hollstein: Valsartan: For years contaminated , Apotheke adhoc, July 10, 2018.
- ↑ NDMA in Valsartan: Another active ingredient producer affected , Pharmazeutische Zeitung, online August 10, 2018, accessed August 29, 2018.
- ↑ NDEA: Second Irbesartan recall, first irbesartan CEP withdrawn , German Apotheker Zeitung, October 11, 2018th
- ↑ a b NDEA Fund: Irbesartan and Losartan lose CEP certificates , Deutsche Apotheker Zeitung, January 21, 2019.
- ^ D. Hüttmann: First Losartan Recalls in Germany , Pharmazeutische Zeitung, January 2, 2019.
- ^ D. Hüttmann: New impurity found in Losartan , Pharmazeutische Zeitung, February 20, 2019.
- ↑ B. Jung: Researchers identify new sartan impurities , Deutsche Apotheker Zeitung, June 5, 2019.
- ↑ a b Thorsten Maybaum: Contaminated antihypertensive drugs: Hundreds of thousands of patients affected , Ärzteblatt, online August 6, 2018, accessed August 29, 2018, pdf aerzteblatt.de .
- ↑ a b Valsartan: EMA expects 1 more cancer case per 5000 patients , Pharmazeutische Zeitung, online August 3, 2018, accessed August 29, 2018.
- ↑ New impurities discovered in sartans apotheke adhoc, June 5, 2019.
- ↑ Sartan medicines: companies to review manufacturing processes to avoid presence of nitrosamine impurities , press release European Medicines Agency. Retrieved March 25, 2019.
- ↑ Implementation decision of the Commission of 2.4.2019 on the authorizations for human medicinal products with the active ingredients "Candesartan", "Irbesartan", "Losartan", "Olmesartan" and "Valsartan" according to Article 31 of Directive 2001/83 / EC of the European Parliament and of Rates (PDF)
- ↑ Sartans: Contamination of the active ingredients, order to suspend approval for some sartan-containing drugs with regard to the avoidance of nitrosamine-containing contamination , BfArM, March 2, 2020.
- ↑ a b c D. Moll: Sartan scandal: EMA publishes limit values for other nitrosamines / , DAZ of September 3, 2019.
- ↑ Temporary interim limits for NMBA, DIPNA and EIPNA impurities in sartan blood pressure medicines , EMA August 20, 2019 (pdf).
- ↑ Not just sartans: Antidiabetic drug contaminated with NDMA , DAZ.online, April 29, 2019.
- ↑ Nitrosamine impurities: NDMA found in ranitidine , DAZ.online, September 13, 2019.
- ↑ EMA to review ranitidine medicines following detection of NDMA , European Medicines Agency, September 13, 2019.
- ^ A. Mende: Recall of all ranitidine preparations , Pharmazeutische Zeitung, April 2, 2020.
- ↑ No metformin recalls for the time being - tests are ongoing , DAZ.online, December 5, 2019.
- ↑ Nitrosamine: EMA develops guidelines for the avoidance of nitrosamine contamination in medicinal products for human use , BfArM, September 17, 2019.
- ↑ EMA to provide guidance on avoiding nitrosamines in human medicines , EMA press release of September 13, 2019