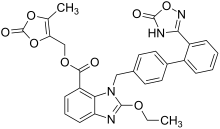
Azilsartan
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
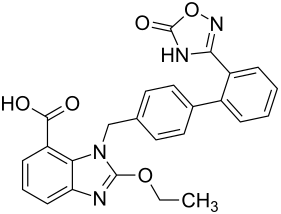
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Azilsartan | |||||||||||||||
| other names |
2-ethoxy-1 - {[2 ′ - (5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl) biphenyl-4-yl] methyl} -1 H -benzimidazol-7- carboxylic acid |
|||||||||||||||
| Molecular formula |
|
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | ||||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Azilsartan is a synthetically produced chemical compound from the group of benzimidazoles that acts as an angiotensin II receptor antagonist and is therefore used as a drug for the treatment of essential hypertension in adults.
Clinical information
Type and duration of application
Therapy with azilsartan can be carried out as monotherapy or as combination therapy with other antihypertensive drugs . The recommended starting dose is 40 mg once a day. If this does not adequately control blood pressure, the dose can be increased to a maximum of 80 mg once daily.
Contraindications
As with other sartans, azilsartan is contraindicated in the second and third trimesters of pregnancy; use in the first trimester is not recommended.
Mechanism of action
The active ingredient is used as the potassium salt of its prodrug azilsartan medoxomil (an ester of the carboxylic acid azilsartan, trade name Edarbi ), which is quickly converted into the active form, azilsartan, after oral administration. The mechanism of action corresponds to that of the other AT 1 antagonists and is described in detail in the article on the active ingredient group of sartans .
Early benefit assessment
An early benefit assessment according to AMNOG is not available for azilsartan. The active ingredient was therefore assigned to a fixed amount group in 2012 .
Individual evidence
- ↑ a b Azilsartan data sheet from Sigma-Aldrich , accessed on May 22, 2017 ( PDF ).
- ↑ a b pharmische-zeitung.de : Azilsartan, Edarbi® (Takeda Pharma)
- ↑ a b c Technical information Edarbi.
- ↑ Federal Joint Committee: Benefit assessment according to Section 35a SGB V - Benefit assessment procedure for the active ingredient Azilsartan Medoxomil , March 15, 2012.