Tetracycline
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Tetracycline | ||||||||||||||||||
| other names |
(4 S , 4a S , 5a S , 6 S , 12a S ) -4- (dimethylamino) -3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a , 5,5a, 6,11,12a-octahydrotetracene-2-carbamide ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 22 H 24 N 2 O 8 | ||||||||||||||||||
| Brief description |
yellow solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 444.44 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
172 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tetracycline or tetracycline is an antibiotic and belongs to the group of tetracyclines . Tetracycline is a broad spectrum antibiotic which is produced by streptomycetes ( Streptomyces aureofaciens ) and is used against many bacterial infections. It is widely used against acne .
synthesis
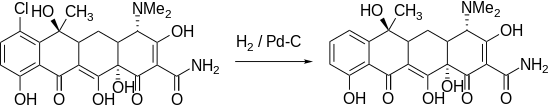
Tetracycline can be obtained from chlorotetracycline by hydrogenation with palladium as a catalyst .
Mechanism of action
It prevents the addition of aminoacyl-tRNA to the rRNA in the 30S subunit of the bacterial ribosome. This stops translation and ultimately protein biosynthesis .
The toxicity could be due to an inactivation of the 30S ribosomes of the mitochondria present in the eukaryotic host cells .
history
The tetracyclines are a large family of antibiotics. In 1948, discovered Benjamin Minge Duggar with chlortetracycline the first tetracycline. He was able to obtain chlortetracycline (7-chloro-TC, CTC) from Streptomyces aureofaciens . Because the agar cultures of Streptomyces aureofaciens were golden yellow in color, the drug was called aureomycin. In the context of the structural elucidation of aureomycin and terramycin, tetracycline was first described in 1952 and in 1953 the first therapeutic results were published with it. The first trade names for tetracycline were achromycin (from Lederle), hostacyclin (from Hoechst ), tetracycline (from Bayer ) and tetracyn (from Pfizer ). Oxytetracycline (5-Hydroxy-TC, OTC) was discovered in extracts of Streptomyces rimosus by Alexander Finlay at Pfizer's research division . Because Streptomyces rimosus was isolated in Terre Haute , IN , USA, OTC was given the trade name Terramycin . By dechlorinating aureomycin, Lloyd Hillyard Conover was able to obtain tetracycline. The tetracycline patent was granted in 1955.
Tetracycline was discovered in Nubian mummies in the 1990s. It is believed that the then-brewed beer could have been a source of tetracycline.
effect
Tetracycline has a bacteriostatic effect on many gram-negative and gram-positive bacteria . Like all tetracyclines, tetracycline inhibits protein synthesis on the bacterial ribosomes.
Therapeutic use
- Infections of the respiratory tract (e.g. atypical pneumonia , drug of choice only for Q fever )
- Infections of the genitourinary system (such as prostatitis )
- Skin infections (such as acne vulgaris )
- Intestinal infections (such as cholera )
- but also plague , brucellosis and tularemia etc.
Other uses
Because tetracycline is absorbed in bone, it is used as a marker of bone growth in human biopsies . The presence of tetracycline in the bone is determined by fluorescence . In Germany this possibility of proof was used until 1998: Tetracycline added to rabies vaccination baits for foxes and deposited in the bones of the animals made it possible to make statements about the intake of the bait. In genetic engineering , tetracycline is used to activate or deactivate transcription in the Tet system developed by Hermann Bujard . This enables genes to be specifically activated and their function to be examined.
Resistance Mechanisms
Bacteria isolated from feces are over 80% resistant to tetracycline. This is due to the following mechanisms:
- Various resistance plasmids encode a tetracycline efflux pump that actively transports the antibiotic (i.e. with ATP hydrolysis) out of the cell
- Ribosome protecting proteins such as Tet (O) can cause resistance by actively removing tetracycline (with GTP hydrolysis) from the ribosome.
- Genomic tetracycline resistance comes about through mutations in the site of action of the antibiotic, the 16S rRNA.
Pharmacokinetics
Oral bioavailability when avoiding prior food intake is given as 60 to 80%. The plasma half-life is 8.5 hours, with excretion mainly via the kidneys, since the tetracycline excreted with the bile is largely reabsorbed from the intestine and is subject to the enterohepatic circulation . The plasma protein binding is 50%.
Contraindications and side effects
- may stain developing teeth (even if taken during pregnancy), see tetracycline teeth
- is mainly inactivated by aluminum , iron and calcium through chelation , therefore do not take it with antacids , iron preparations or dairy products
- phototoxic , therefore no strong sun exposure under tetracycline
- not to be taken during pregnancy
- not for children under 8 years
- do not take in case of tetracycline allergy
Chemical properties
Tetracycline hydrochloride, a yellow, crystalline, hygroscopic powder turns brown through direct contact with sun rays and when stored in moist air.
- dissociation
- The hydrochloride can release 3 protons in an aqueous solution. Relevant to the equilibrium reactions pK s values are 3.3; 7.7; 9.7. A 2% aqueous solution has a pH of approx. 2.2.
Trade names
Monopreparations : Actisite-Dental (A), Imex (D), Tefilin (D), as well as generics
- With amphotericin B : Mysteclin (D, A)
- with citric acid - bismuth - potassium salt and metronidazole : Pylera (D, A)
- Veterinary medicine
Cyclutrin, Tetrabiotic, Tetran, U-tab, Utroletten
Individual evidence
- ↑ a b c Datasheet Tetracycline from Sigma-Aldrich , accessed on October 23, 2016 ( PDF ).
- ↑ a b c Entry on tetracycline in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ a b Entry on tetracyclines. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ Axel Kleemann, Jürgen Engel: Active pharmaceutical ingredients: syntheses, patents, applications . 2nd Edition. Thieme, Stuttgart, New York 1982, ISBN 3-13-558402-X , p. 864-865 .
- ^ Benjamin Minge Duggar: Aureomycin: A Product of the Continuing Search for New Antibiotics. In: Annals of the New York Academy of Sciences . tape 51 , 1948, p. 177-181 , doi : 10.1111 / j.1749-6632.1948.tb27262.x , PMID 18112227 .
- ↑ Karl Wurm, AM Walter: Infectious Diseases. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition, ibid. 1961, pp. 9–223, here: p. 50.
- ^ Mark L Nelson, Stuart B Levy: The history of the tetracyclines . In: Annals of the New York Academy of Sciences . tape 1241 , December 2011, p. 17-32 , doi : 10.1111 / j.1749-6632.2011.06354.x , PMID 22191524 .
- ↑ Patent US2699054 .
- ↑ Take Two Beers and Call Me in 1,600 Years - use of tetracycline by Nubians and Ancient Egyptians ( Memento from May 29, 2012 in the web archive archive.today )
- ↑ S3 guideline on epidemiology, diagnostics, antimicrobial therapy and management of adult patients with community-acquired deep respiratory infections of the German Society for Pneumology and Respiratory Medicine . In: AWMF online (July 2009)
- ↑ Cathy Mayton: Tetracycline labeling of bone .
- ↑ Michael T. Madigan, John M. Martinko, Kelly Bender: Brock Biology of Microorganisms .
- ↑ a b c Pharmacopoeia Commentary. Complete works including 36th update delivery 2010, ISBN 978-3-8047-2461-7 .