Simvastatin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
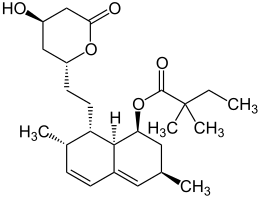
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Simvastatin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 25 H 38 O 5 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 418.57 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
127-132 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Simvastatin is the non-proprietary name of a drug from the group of statins that is used as a cholesterol-lowering drug .
Simvastatin is structurally derived from the naturally occurring monacolin K ( lovastatin ).
Mechanism of action
Simvastatin is an HMG-CoA reductase inhibitor. The HMG-CoA reductase acts as a catalyst in the reduction of 3-hydroxy-3-methylglutaryl-coenzyme A to mevalonate, which is a limiting step in the hepatic cholesterol synthesis. By lowering the cholesterol synthesis, the liver cells increase the number of LDL receptors on the cell surface, so that the LDL uptake into the liver cell increases and thus the LDL level in the blood is reduced.
Absorption and distribution in the body
After ingestion, the active ingredient is mainly metabolized by the cytochrome P450 3A4 , therefore CYP – 3A4– inhibitors such as ketoconazole , itraconazole and clarithromycin or grapefruit juice should not be taken together with simvastatin.
Side effects
Simvastatin has the usual side effects of this group of active substances , such as upper abdominal discomfort, increased transaminases and myopathies such as rhabdomyolysis .
Manufacturing
It is produced partially synthetically from lovastatin , with its 2-methylbutanoyl side chain being transesterified to give the 2,2-dimethylbutanoyl radical.
A multi-step synthesis for simvastatin is described in the literature.
Forms of trade
Simvastatin was launched in Germany in 1990 by MSD Sharp & Dohme as Zocor . In May 2003 the patent on the active ingredient expired. Since then, simvastatin has also been produced and sold by various generic manufacturers. Tablets are available in doses of 5, 10, 20, 30, 40, 60 and 80 mg.
Zocor (D, CH), Zocord (A), numerous generics (D, A, CH)
with ezetimibe : Inegy (D, A, CH), Goltor
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Simvastatin
- Inegy on the me-too list 2016 (PDF)
Individual evidence
- ↑ a b c d data sheet Simvastatin from Sigma-Aldrich , accessed on June 13, 2011 ( PDF ).
- ↑ Information from Merck (PDF; 166 kB).
- ↑ Richard Daikeler, idols Use, Sylke Waibel: diabetes. Evidence-based diagnosis and therapy. 10th edition. Kitteltaschenbuch, Sinsheim 2015, ISBN 978-3-00-050903-2 , p. 149.
- ↑ K. Hardtke et al. (Ed.): Commentary on the European Pharmacopoeia Ph. Eur. 6.4, Simvastatin. Loose-leaf collection, 34th delivery 2010, Wissenschaftliche Verlagsgesellschaft Stuttgart.
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dietmar Reichert: Pharmaceutical Substances, 4th edition (2000), 2 volumes published by Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 ; online since 2003 with biannual additions and updates.
- ↑ Red List online, as of September 2009.
- ↑ AM comp. d. Switzerland, as of September 2009.
- ↑ AGES-PharmMed, as of September 2009.