Cytochrome P450 3A4
| Cytochrome P 450 3A4 | ||
|---|---|---|
|
||
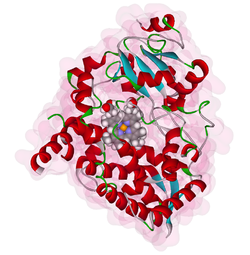
| Structural model of cytochrome P 450 3A4 with central heme group according to PDB 1W0E | ||
| Properties of human protein | ||
| Mass / length primary structure | 503 AS ; 57.3 kDa | |
| Cofactor | Hamm | |
| Identifier | ||
| Gene name | CYP3A4 | |
| External IDs | ||
| Enzyme Classifications | ||
| EC, category | 1.14.13.32 , monooxygenase | |
| Response type | oxidation | |
| Substrate | Albendazole + NADPH / H + + O 2 | |
| Products | Albendazole S-oxide + NADP + + H 2 O | |
| EC, category | 1.14.13.67 , monooxygenase | |
| Response type | Hydroxylation | |
| Substrate | Quinine + NADPH / H + + O 2 | |
| Products | 3-hydroxyquinine + NADP + + H 2 O | |
| EC, category | 1.14.13.97 , monooxygenase | |
| Response type | Hydroxylation | |
| Substrate | Lithocholate + NADPH / H + + O 2 | |
| Products | Hyocholate + NADP + + H 2 O | |
| Occurrence | ||
| Parent taxon | Vertebrates | |
Cytochrome P 450 3A4 (abbreviated: CYP 3A4 ) is an isoenzyme of the cytochrome P 450 superfamily . In the human body, it is one of the central components of metabolism ( biotransformation ), particularly of exogenous substances ( xenobiotics ). In terms of quantity, cytochrome P 450 3A4 occurs most in the liver. It has the most substrates of all cytochromes. Since many drugs are also broken down via cytochrome P 450 3A4, it is the center of many drug interactions . It is the most abundant cytochrome P 450 and metabolizes about half of all drugs.
genetics
In humans, cytochrome P 450 on the 3A4 CYP3A4 - gene coded. This gene is located on chromosome 7q21.1 along with many other cytochrome genes.
function
Cytochrome P 450 enzymes are almost exclusively monooxygenases that catalyze hydroxylation. In addition to the synthesis of steroids and steroid hormones, this reaction is important in biotransformation in order to make hydrophobic substances water-soluble and thus excretable. In addition, it plays a major role in drugs that are administered as prodrugs . The active form is formed here by enzyme catalysis. When CYP 3A4 is inhibited, the active drug is not produced or is produced only to a limited extent.
Because many drugs are broken down via the same isoenzyme, there are particularly many drug interactions there. Some substances induce the enzyme, others inhibit it. The substances broken down by it can accumulate through an enzyme inhibition, which can go so far that the toxic threshold is exceeded. With enzyme induction, a substrate can be broken down to such an extent that the blood plasma effective level is no longer reached.
Substrates
- Immunosuppressants : tacrolimus , ciclosporin , sirolimus
- Chemotherapeutic agents : Cyclophosphamide , Erlotinib , Gefitinib , Doxorubicin , Etoposide , Vindesine , Vinblastine , Tamoxifen
- Antifungal drugs : clotrimazole , ketoconazole , itraconazole
- Antibiotics : metronidazole
- Macrolides : clarithromycin , erythromycin
- tricyclic antidepressants : amitriptyline , clomipramine , imipramine
- SSRIs : citalopram , escitalopram , fluoxetine and norfluoxetine , sertraline
- Buspirone ( anxiolytic )
- Venlafaxine ( SNRI )
- Antipsychotics : aripiprazole , haloperidol , pimozide , risperidone , ziprasidone
- Opioids : alfentanil , codeine , fentanyl , methadone
- Benzodiazepines : Alprazolam , Clonazepam , Flunitrazepam , Midazolam , Triazolam
- Statins : atorvastatin , lovastatin , simvastatin
- Calcium channel blockers : amlodipine , diltiazem , felodipine , nifedipine , verapamil
- Antiarrhythmics : dronedarone , amiodarone
- PDE-5 inhibitors : sildenafil , tadalafil
Inductors
- Hyperforin in St. John's wort
- ginger
- garlic
- liquorice
- Rifampicin ( antibiotic )
- Anticonvulsants : phenytoin , carbamazepine , oxcarbazepine
- phenobarbital
- Modafinil
Inhibitors
- 6 ', 7'-dihydroxybergamotine , bergamotine and naringin in grapefruit juice
- Turmeric
- ginseng
- many antibiotics : macrolides ( erythromycin , clarithromycin , telithromycin ), metronidazole , chloramphenicol , fluoroquinolones
- Antifungal drugs ( fluconazole , ketoconazole , itraconazole )
- Protease inhibitors from HIV therapy ( ritonavir , indinavir , nelfinavir )
- Aprepitant ( antiemetic )
- Verapamil ( calcium channel blocker )
- Nefazodone ( antidepressant )
Examples of interactions
An organ transplantation requires immunosuppression a transplant rejection to prevent. Most immunosuppressants are metabolized by CYP3A4, which means that taking St. John's wort at the same time , e.g. B. in the context of a mild depressive episode, leads to a decrease in the plasma level of the immunosuppressant and can possibly lead to a rejection reaction with organ failure.
Web links
- The cytochrome P450 superfamily on KEGG
- Indiana University - School of Medicine: List of Metabolized Drugs, Enzyme Inducers, and Enzyme Inhibitors for Each Isoenzyme
- Drug interactions
Individual evidence
- ↑ FP Guengerich: Cytochrome P-450 3A4: regulation and role in drug metabolism. In: Annual review of pharmacology and toxicology. Volume 39, 1999, pp. 1-17, doi : 10.1146 / annurev.pharmtox.39.1.1 , PMID 10331074 .
- ↑ Hashimoto H, Toide K, Kitamura R, Fujita M, Tagawa S, Itoh S, Kamataki T: Gene structure of CYP3A4, an adult-specific form of cytochrome P 450 in human livers, and its transcriptional control . In: Eur. J. Biochem. . 218, No. 2, December 1993, pp. 585-95. doi : 10.1111 / j.1432-1033.1993.tb18412.x . PMID 8269949 .
- ↑ Inoue K, Inazawa J, Nakagawa H, Shimada T, Yamazaki H, Guengerich FP, Abe T: Assignment of the human cytochrome P-450 nifedipine oxidase gene (CYP3A4) to chromosome 7 at band q22.1 by fluorescence in situ hybridization . In: Jpn. J. Hum. Genet. . 37, No. 2, June 1992, pp. 133-8. doi : 10.1007 / BF01899734 . PMID 1391968 .
- ↑ Druglib.com
- ↑ a b Thomas Effert: Molecular Pharmacology and Toxicology: Biological Foundations of Drugs and Poisons, p. 24 . Springer, Berlin 2006, ISBN 978-3-540-21223-2 .
- ↑ Pharmaceutical newspaper online 35/2008: Grapefruit - Discovery of new mechanism of interaction. Retrieved January 10, 2011 .
- ↑ a b c d e Björn Lemmer , Georges Fülgraff (ed.): Pharmacotherapy, clinical pharmacology . 14., revised. and updated edition. Springer, Heidelberg 2010, ISBN 978-3-642-10540-1 , pp. 470 .
- ↑ Park JY, Kim KA, Kim SL: Chloramphenicol is a potent inhibitor of cytochrome P 450 isoforms CYP2C19 and CYP3A4 in human liver microsomes . In: Antimicrob. Agents Chemother. . 47, No. 11, November 2003, pp. 3464-9. doi : 10.1128 / AAC.47.11.3464-3469.2003 . PMID 14576103 . PMC 253795 (free full text).