Venlafaxine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
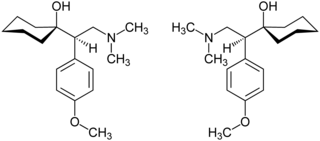
| ( R ) -Venlafaxine (left) and ( S ) -Venlafaxine (right) enantiomers (1: 1 mixture) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Venlafaxine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 17 H 27 NO 2 | |||||||||||||||||||||
| Brief description |
white to almost white, polymorphic powder (hydrochloride) |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 277.40 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
215–217 ° C (venlafaxine hydrochloride ) |
|||||||||||||||||||||
| solubility |
Easily soluble in water and methanol , soluble in absolute ethanol , hardly soluble to practically insoluble in acetone (venlafaxine hydrochloride ) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Venlafaxine is a drug that is used in the treatment of depression and anxiety disorders . Chemically, it is a phenylethylamine - derivative that as a selective serotonin-norepinephrine reuptake inhibitor its effect in the central nervous system develops.
Action profile
Venlafaxine reduces the reuptake of serotonin and noradrenaline into the presynaptic vesicles at certain synapses in the brain . The resulting increased supply of these neurotransmitters in the synaptic gap is intended to alleviate the depressive symptoms .
Venlafaxine inhibits the serotonin transporter with about 30 times the affinity compared to the norepinephrine transporter and also inhibits dopamine reuptake, albeit very weakly. In practice, this means that venlafaxine in low doses is more like an SSRI and its noradrenergic component only comes into play from higher doses.
It therefore has the greatest selectivity for serotonin within the group of serotonin-norepinephrine reuptake inhibitors. While duloxetine shows a 10-fold greater selectivity for serotonin in relation to noradrenaline, milnacipran blocks the serotonin and noradrenaline reuptake about equally.
The absolute bioavailability of venlafaxine is only 40% to 45%, which is due to the considerable metabolism in the liver . Venlafaxine is primarily metabolized via the CYP2D6 to the active metabolite O -desmethylvenlafaxine, to a much lesser extent via the CYP3A4 to the less active secondary metabolite N -desmethylvenlafaxine. Excretion occurs primarily via the kidneys (renal). The half-lives are 5 hours for venlafaxine and 11 hours for O- desmethylvenlafaxine.
The peak plasma concentrations of venlafaxine and O -Desmethylvenlafaxin be in immediate release tablets or capsules within 2 or 3 hours and at sustained release ( retarded ) forms reached within 5.5 and 9 hours.
Indications
Medicines containing venlafaxine are indicated for the treatment of depression and anxiety disorders such as generalized anxiety disorder , social anxiety disorder ( social phobia ) and panic disorder (with or without agoraphobia ). Venlafaxine is also indicated for maintenance therapy to prevent relapse ( relapse prophylaxis) or the recurrence of new depressive illnesses. It is also used in the context of off-label use for the treatment of diabetic polyneuropathy . Even in the context of ADHD treatment, failed attempts with other stimulants, such as B. methylphenidate , achieve success because it also has a stimulating effect at higher doses.
For girls or boys from 9 to 18 years of age with moderate to severe depression - but not with subliminal or extremely severe depression - it has not been proven that venlafaxine or any of the other 13 antidepressants works better than placebos - with the exception of fluoxetine , psychiatrists around Andrea warn Cipriani (Oxford University) in the “Lancet” based on the findings on 5260 test persons: Venlafaxine was associated with a significantly increased risk of suicidal thoughts and actions. In some of the 34 studies, unpleasant results were withheld. Some manufacturers even refused to provide anonymized patient data.
unwanted effects
Gastrointestinal complaints often occur as side effects at the beginning of use ; there is often increased restlessness and diffuse anxiety states. Psychotic reactions have been described, possibly as a result of the dopaminergic effect. Increased (night) sweating, increased blood pressure and heart problems are also possible. Nausea is a very common side effect, especially at the beginning of treatment (more than 10% of patients); vomiting, loss of appetite, constipation, dizziness, insomnia, nervousness, grinding of teeth, tremors and visual disturbances are common (1–10% of patients). It can also lead to drowsiness and fatigue. Venlafaxine very often causes sexual dysfunction in men (ejaculation difficulties) and can reduce libido .
Suicidality
According to an analysis of US regulatory FDA from 2006, all data from clinical trials of venlafaxine considered (both published and from unpublished studies), venlafaxine can in under 25 users to suicidality compared to taking dummy tablets ( placebo ) Increase by a factor of 5. A placebo-controlled US meta-study from 2012 with 9185 patients could not find a connection between the administration of SSRIs, here fluoxetine and venlafaxine, and an increased risk of suicide. The study comes to the conclusion that the assumptions of the American health authority FDA, which led to the warning labels, are incorrect.
Because of the alleged particular risk of suicide and the higher toxicity compared to other antidepressants , according to some experts, venlafaxine should no longer be used for the first treatment of depression. In the UK, the guidelines on the treatment of depression have been revised to take into account the particular risks of venlafaxine. In Germany, a step-by-step plan procedure made it mandatory to include a corresponding warning in the product information.
Dosage forms
Venlafaxine is orally administered, there are tablets or capsules to 37.5 mg and 75 mg or so-called. Retard formulations to 37.5 mg, 75 mg, 150 mg and 225 mg, which release the drug delayed. Because of the occasional occurrence of gastrointestinal complaints, the retard form is predominantly prescribed in Germany. Venlafaxine is used as a hydrochloride for the production of the drug formulations .
In good time before the patent expiry of venlafaxine, the manufacturer Wyeth tried to bring a new dosage form onto the market and in September 2007 submitted an EU-wide (central) application for approval for desvenlafaxine (the active metabolite O-desmethylvenlafaxine). However, the European authority had concerns as the effectiveness of desvenlafaxine could not be convincingly demonstrated. Compared to the parent compound venlafaxine, desvenlafaxine appears to be less effective and to offer no advantage in terms of safety and tolerability. In addition, the Scientific Panel ( CHMP ) considered the data on the short-term and long-term effects of desvenlafaxine to be insufficient. Wyeth then withdrew the application.
In the USA, however, desvenlafaxine is on the market as Pristiq .
Use during pregnancy and breastfeeding
Patients should tell their doctor if they are pregnant or planning to become pregnant. Venlafaxine may only be used during pregnancy if there are compelling indications and only if prescribed by a doctor. The following symptoms may appear immediately or shortly after birth in newborns whose mothers received venlafaxine in the late stages of pregnancy: eating and sleeping disorders, breathing difficulties, seizures, difficulty regulating body temperature, low blood sugar levels, tremors, tense or excessive relaxed muscles, vomiting, irritability and constant crying. If a newborn has any of these symptoms, a doctor should be contacted.
In an observational study (breastfed children of mothers who were treated with venlafaxine) none of the children examined showed symptoms that could be attributed to the drug. The relative dose is 6–8% including the major metabolite desmethylvenlafaxine. Only the metabolite could be detected in the serum of breastfed children.
At present, breastfeeding appears to be acceptable with maternal monotherapy with venlafaxine if the child is closely observed. The decision for or against breastfeeding must be made individually.
Interactions with other drugs
Venlafaxine is a substrate of the cytochrome P450 2D6 and can slow down the metabolism of other substances (due to its only slightly inhibitory effect on this enzyme compared to other antidepressants) , which can lead to poisoning. Venlafaxine is differently effective in patients with different cytochrome P450 variants. As with other serotonergic agents, treatment with venlafaxine can lead to a potentially life-threatening condition called serotonin syndrome . This is especially the case when other substances are ingested that can affect the serotonergic neurotransmitter system. These include, for example, St. John's wort , lithium , triptans , serotonin reuptake inhibitors (SSRI), sibutramine and tramadol . Venlafaxine must not be taken together with irreversible and should not be taken together with reversible monoamine oxidase inhibitors (MAOIs). A sufficiently long therapy-free interval must be observed when changing medication. Concomitant use of venlafaxine with CYP3A4 inhibitors such as atazanavir , clarithromycin , indinavir , itraconazole , voriconazole , posaconazole , ketoconazole , nelfinavir , ritonavir , saquinavir, and telithromycin can also increase venlafaxine levels.
Venlafaxine can increase the AUC of haloperidol , risperidone, and metoprolol . The AUC of indinavir is reduced with concomitant use of venlafaxine. The clinical relevance of these interactions is unknown.
Withdrawal syndrome
Due to the possible occurrence of withdrawal syndrome, it is recommended that treatment be gradually discontinued, i.e. that the dose of venlafaxine be reduced gradually and the patient monitored. The frequency of symptoms depends on the dose, the duration of treatment and the individual patient. The following symptoms have been reported in association with abrupt discontinuation, dose reduction, or tapering of treatment: loss of appetite ( anorexia ), feelings of anxiety, urge to move ( agitation ), confusion, diarrhea , drowsiness, dry mouth, tiredness, headache, hypomania , insomnia or other sleep disorders Nervousness, paresthesia , drowsiness, sweating, dizziness , nausea , vomiting, tremors ( tremor ), nightmares, weakness, hyperacusis , changes in taste, distorted vision, confusion (confusion), ego disorders ( depersonalization ), noises in the ears ( tinnitus ), sensory disorders such as especially “brain zaps”, delusional ideas and distorted perception.
The majority of withdrawal symptoms are not severe and resolve spontaneously, usually within two weeks of stopping venlafaxine. In individual cases, they can last two to three months or longer.
chemistry
Stereoisomerism
Venlafaxine is chiral and contains a stereocenter. There are thus two enantiomers , the ( R ) form and the ( S ) form. The commercial preparations contain the drug as a racemate (1: 1 mixture of the enantiomers), whereby the use of the better and at the same time mostly less side effects effective enantiomer ( eutomer ) would be preferable for basic considerations .
Manufacturing
The synthesis of racemic venlafaxine starts with cyclohexanone and 4-methoxyphenylacetonitrile .
The nitrile alcohol is obtained by deprotonating the nitrile in the α-position and an aldol-like reaction . The catalytic hydrogenation provides the amino alcohol. The active ingredient is finally obtained by dimethylation in an Eschweiler-Clarke reaction .
Structure-activity relationships
An original analogue is ( R ) -Sila-Venlafaxine, which no longer shows any activity on SERT, retains it on NAT and has an antiemetic effect.
Trade names
Efectin (A, PL), Efexor (CH, DK, ES, FI, IT, NL, S, UK), Effexor (CDN, F, USA), Trevilor retard (D), numerous generics
Web links
Individual evidence
- ↑ a b European Pharmacopoeia Commission (ed.): EUROPEAN PHARMACOPOE 5TH EDITION . tape 5.0-5.8 , 2006.
- ↑ a b entry on venlafaxine. In: Römpp Online . Georg Thieme Verlag, accessed on November 11, 2014.
- ↑ a b data sheet Venlafaxine hydrochloride from Sigma-Aldrich , accessed on April 25, 2011 ( PDF ).
- ↑ S. Stahl et al. SNRIs: Their Pharmacology, Clinical Efficacy, and Tolerability in Comparison with Other Classes of Antidepressants. CNS Spectr. 2005; 10 (9): 732-47 .
- ↑ Moret C, Charveron M, Finberg JP, Couzinier JP, Briley M: Biochemical profile of midalcipran (F 2207), 1-phenyl-1-diethyl-aminocarbonyl-2-aminomethyl-cyclopropane (Z) hydrochloride, a potential fourth generation antidepressant drug . In: Neuropharmacology . 24, No. 12, 1985, pp. 1211-9. doi : 10.1016 / 0028-3908 (85) 90157-1 . PMID 3005901 .
- ↑ E. Mutschler , G. Geisslinger, Heyo K. Kroemer , P. Ruth, M. Schäfer-Korting: drug effects. Textbook of pharmacology and toxicology. 9th edition. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 2008, ISBN 3-8047-1952-X .
- ↑ German Society for Neurology, Therapy of Neuropathic Pain, as of 2008 (PDF file; 180 kB) .
- ↑ Andrea Cipriani, Xinyu Zhou et al. a .: Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. In: The Lancet. 2016, doi : 10.1016 / S0140-6736 (16) 30385-3 .
- ^ AL Montejo, G. Llorca, JA Izquierdo, F. Rico-Villademoros: Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. In: The Journal of clinical psychiatry. Volume 62 Suppl 3, 2001, pp. 10-21, PMID 11229449 .
- ↑ U. Werneke, S. Northey, D. Bhugra: Antidepressants and sexual dysfunction. In: Acta Psychiatrica Scandinavica. 114, 2006, p. 384, doi : 10.1111 / j.1600-0447.2006.00890.x .
- ^ Clinical Review : Relationship between Antidepressant Drugs and Suicidality in Adults . FDA, December 5, 2006 (PDF; 2 MB).
- Jump up ↑ Robert D. Gibbons, C. Hendricks Brown, Kwan Hur, John M. Davis, J. John Mann: Suicidal thoughts and behavior with antidepressant treatment reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine.
- ↑ Süddeutsche.de: Risk of suicide in adolescents: all-clear for antidepressants
- ↑ A. Cipriani, J. R Geddes, C. Barbui: Venlafaxine for major depression. In: BMJ. 334, 2007, p. 215, doi : 10.1136 / bmj.39098.457720.BE .
- ^ NICE - Consultation: Amendment to clinical guideline on depression (clinical guideline 23) ( Memento of April 30, 2007 in the Internet Archive ).
- ↑ Federal Institute for Drugs and Medical Devices (BfArM): Antidepressants: Suicidality in young adults .
- ↑ Withdrawal EPAR Desvenlafaxine ( Ellefore ) (PDF; 260 kB).
- ↑ FDA Pristiq .
- ↑ Pharmacovigilance and Counseling Center for Embryonic Toxicology. Retrieved July 15, 2011.
- ↑ Torsten Kratz, Albert Diefenbacher: Psychopharmacotherapy in old age. Avoidance of drug interactions and polypharmacy. In: Deutsches Ärzteblatt. Volume 116, Issue 29 f. (July 22) 2019, pp. 508-517, p. 509.
- ^ Indiana University: Cytochrome P450 Drug Interaction Table . PDF .
- ↑ DE McAlpine, JM Biernacka, DA Mrazek, DJ O'Kane, SR Stevens, LJ Langman, VL Courson, J. Bhagia, TP Moyer: Effect of cytochrome P450 enzyme polymorphisms on pharmacokinetics of venlafaxine. In: Therapeutic Drug Monitoring . Volume 33, Number 1, February 2011, pp. 14-20, doi : 10.1097 / FTD.0b013e3181fcf94d . PMID 21099743 .
- ↑ German specialized information: Effexor retard, as of May of 2007.
- ↑ Information for professionals of the Swiss Medicines Compendium: Efexor ; Information as of August 2013.
- ↑ ROTE LISTE 2017, Verlag Rote Liste Service GmbH, Frankfurt am Main, ISBN 978-3-946057-10-9 , p. 225.
- ↑ Everhardus Ariëns : Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology , European Journal of Clinical Pharmacology 26 (1984) 663-668, doi : 10.1007 / BF00541922 .
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dietmar Reichert: Pharmaceutical Substances , 4th edition (2000), 2 volumes published by Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 ; online since 2003 with biannual additions and updates.
- ↑ Showell GA, Barnes MJ, Daiss JO, et al : (R) -sila-venlafaxine: a selective noradrenaline reuptake inhibitor for the treatment of emesis . In: Bioorg. Med. Chem. Lett. . 16, No. 9, 2006, pp. 2555-2558. doi : 10.1016 / j.bmcl.2005.12.062 . PMID 16513343 .
