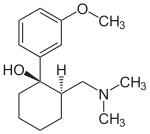
Tramadol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| 1: 1 mixture of (1 R , 2 R ) -tramadol (left) and (1 S , 2 S ) -tramadol (right) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Tramadol | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 16 H 25 NO 2 | |||||||||||||||||||||
| Brief description |
white to almost white, crystalline powder (hydrochloride) |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 263.38 g · mol -1 | |||||||||||||||||||||
| pK s value |
9.41 (hydrochloride) |
|||||||||||||||||||||
| solubility |
slightly soluble in water and methanol , very slightly soluble in acetone (hydrochloride) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Tramadol is a drug belonging to the group of opioids and is used to treat moderate to severe pain . The substance was developed synthetically by Grünenthal GmbH and brought onto the market in 1977 as a drug (Tramadol- HCl ) under the name Tramal .
history
The Grünenthal company in Stolberg commissioned the then employee Kurt Flick to develop a new cough blocker ( antitussive ). In 1962 he synthesized the isomeric mixture "L / 201", which in the pharmacological test, a high analgesic ( analgesic having) effect and was then applied for patent. Flick's successor Ernst Frankus (1928–1995) succeeded in isolating the trans isomer tramadol by isomeric separation , which was patented by Grünenthal in 1977.
effect
Tramadol is an agonist of the μ, δ and κ opioid receptors in nervous tissue. However, the affinity is low and shows no particular specificity for the individual opioid receptors. The dampening of pain perception is therefore also mediated by other mechanisms: by inhibiting the reuptake of norepinephrine into the neuron and increasing the release of serotonin by the (-) - enantiomer . This mechanism of action also explains the slightly antidepressant and anxiolytic (anxiety-relieving and calming) effect. The increased occurrence of nausea as an undesirable effect is also explained by the increased release of serotonin.
The analgesic potency is one tenth that of morphine . Tramadol is next meptazinol and nalbuphine one of the three approved injectable opioid - analgesics that are not in Germany under the Narcotics Act fall.
The two enantiomers of tramadol [(1 R , 2 R ) -tramadol and (1 S , 2 S ) -tramadol] and their metabolites , in particular the derivatives demethylated on oxygen (nortramadole), have pharmacological effects of different strengths [μ-opioid- Bond K i (μM)]:
- (1 R , 2 R ) -Tramadol: 5.1
- (1 S , 2 S ) -Tramadol: 120
- (1 R , 2 R ) -Nortramadol: 0.02
- (1 S , 2 S ) -Nortramadol: 1.8
Tramadol is approximately 95% absorbed after oral administration. The oral bioavailability is given as 60 to 75% and the plasma half-life as around 5 to 6 hours.
The analgesic duration of action (a single dose of 50 to 100 mg) is 2 to 4 hours, and for sustained-release preparations 8 to 12 hours.
application areas
Tramadol is indicated for the treatment of moderate to severe pain and can be administered orally (as a capsule of 50 mg, drops (50 mg = 20 drops) or an extended-release tablet containing 100 to 200 mg of active ingredient), rectally as a suppository (100 mg) and intravenously (as Solution for injection with a concentration of 50 mg / ml).
Outside of the approved areas of application, tramadol is used in so-called off-label use for the treatment of restless legs syndrome .
Another off-label use is the treatment of ejaculatio praecox ( premature ejaculation ).
In Germany, tramadol drops are also approved for children from one year of age in the case of severe pain, the dosage being based on body weight. The correct dosage is guaranteed by the container's drip device.
In Australia, on the other hand, tramadol in the form of drops is not approved for children under 12 years of age. In this country there are no instructions on caution and dosage for this patient group. Since the strength of the drug was underestimated, with sometimes serious consequences, the Australian Medicines Agency Therapeutic Goods Administration (TGA) warns against such off-label use .
Side effects
Side effects such as sweating, sedation, and confusion can occur, as can difficulty urinating , drowsiness, and blurred vision. In therapeutic doses, tramadol has no significant influence on respiration and pulmonary artery pressure due to its low μ-selectivity . Severe nausea is often observed, both with oral administration and with too rapid injection. Blood pressure and pulse rate are hardly affected. Seizures have been reported, especially when given at doses above the therapeutic dose. Because tramadol is excreted in breast milk, the New Zealand Medicines Agency issued a warning that the active ingredient could harm babies . The World Health Organization (WHO) Pharmacovigilance Center reported a risk signal for hyperacusis with tramadol in 2020. Three quarters of those affected are women. Where indicated, most of the hypersensitivity to normal volume sounds occurs within one day of ingestion.
More recent studies also show an increased risk of severe hypoglycaemia and hyponatremia , some of which are fatal.
Analytics
The reliable determination of tramadol in different test material such as blood serum , blood plasma , urine or hair is possible after appropriate sample preparation by coupling gas chromatography or HPLC with mass spectrometry .
Interactions with other drugs
Pharmacological
Tramadol, when used with bupropion and MAOIs, can develop serious side effects.
Interactions also occur with oral anticoagulants, alcohol, benzodiazepines (depression of the respiratory center up to possible respiratory arrest ) and serotoninergic substances (danger of serotonin syndrome ). Serotoninergic substances include SSRI antidepressants such as B. fluoxetine and citalopram and also illegal drugs like ecstasy and cocaine . Over-the-counter preparations made from St. John's wort (St. John's wort tea, St. John's wort extract in capsules, etc.) can also trigger a serotonin syndrome.
Chemico-physical
Tramadol injection solutions are incompatible with parenteral dosage forms of diazepam , diclofenac , flunitrazepam , glycerol trinitrate , indomethacin , DL- lysine monoacetyl salicylate , midazolam , piroxicam and phenylbutazone if they are drawn up in the same syringe; flocculation occurs .
Habituation and potential for dependence
As an agonist (among others) of the μ-opioid receptor, there is basically a potential for dependency, especially in the case of improper use. In general, the dose should be titrated against the pain according to the principle “as little as possible, as much as necessary”.
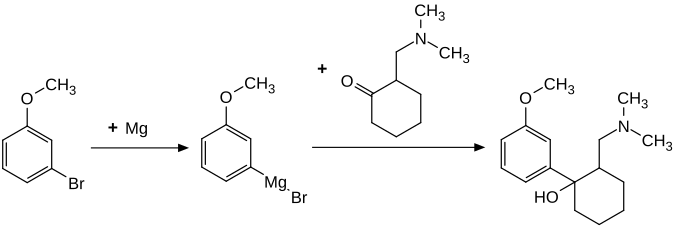
Chemistry and isomerism
Tramadol is produced synthetically on an industrial scale. The two-step chemical synthesis is based on 3-bromanisole , the Grignard compound of which is converted into the target compound by reaction with racemic 2-dimethylaminomethylcyclohexanone:
Tramadol [2- (dimethylaminomethyl) -1- (3-methoxyphenyl) cyclohexanol] has two stereogenic centers on the cyclohexane ring . There are four configurational isomers of 2- (dimethylaminomethyl) -1- (3-methoxyphenyl) cyclohexanol :
- (1 R , 2 R ) shape,
- (1 S , 2 S ) shape,
- (1 R , 2 S ) shape and the
- (1 S , 2 R ) shape.
The synthesis produces the (1 R , 2 R ) form and the (1 S , 2 S ) form as the main product in the same amount. The racemate from the (1 R , 2 S ) form and the (1 S , 2 R ) form is formed in smaller quantities during the synthesis . The isolation of the (1 R , 2 R ) form and (1 S , 2 S ) form and thus the separation of the by-product racemate from (1 R , 2 S ) form and the (1 S , 2 R ) - Form is achieved through the fractional crystallization of the hydrochloride . Tramadol is used medicinally as a racemate from the (1 R , 2 R ) form and the (1 S , 2 S ) form in the form of its hydrochloride. The (1 R , 2 R ) form is also called (+) - tramadol, the (1 S , 2 S ) form (-) - tramadol.
The separation of the racemate from the (1 R , 2 R ) form and the (1 S , 2 S ) form with ( R ) - or ( S ) - mandelic acid is described in the literature, but is not used industrially because Tramadol is used as a mixture of enantiomers , although the different physiological effects of the (1 R , 2 R ) and (1 S , 2 S ) enantiomers have been proven.
The hydrochloride of the racemate from the (1 R , 2 R ) form and the (1 S , 2 S ) form, which Grünenthal developed as a medicinal substance in Germany, was incorrectly identified in the original patent and in the vast majority of publications as (±) - trans -tramadol described. In the course of the approval process in the United States, the name was changed to (±) - cis -Tramadol. Alternatively, the racemic drug (±) - cis -tramadol can also be referred to as (±) - (1 R *, 2 R *) - tramadol, with (1 R *, 2 R *) indicating the relative stereochemistry, es is therefore a 1: 1 mixture of the (1 R , 2 R ) form and the (1 S , 2 S ) form.
Tramadol as an apparent natural substance
In 2013, scientists from France, Switzerland and Cameroon published the discovery that tramadol is contained in the root bark of the African medicinal plant Nauclea latifolia . However, further investigations in 2014 by German and Cameroonian scientists showed that the occurrence of tramadol in Nauclea latifolia is a result of the fact that cattle in the region concerned are regularly treated with the active ingredient, leading to cross-contamination of the soil with urine and faeces The animals come and the plant absorbs the tramadol from it and accumulates it.
Trade names
Monopreparations
Adamon (A), Amadol (D), Contramal (A), Cromatodol (A), Ecodolor (CH), Jutadol (D), Lanalget (A), Noax (A), Nobligan (A), Tramal-long (D ), Tradolan (A, S), Tradonal (CH), Tramagit (D), Tramal (D, A), Tramundin and Tramundin retard (D, CH), Travex (D), Ultram, numerous generics (D, A, CH)
Combination preparations
With paracetamol : Dolevar (D), Zaldiar (D, A, CH, B), as well as generics of z. B. TAD, Stada and Hexal
Dissemination and Abuse
Tramadol is used as a drug by large parts of the population in West Africa . It comes mainly from Indian generic drug manufacturers. According to a report from April 2018, according to have been in the past five years in Africa south of the Sahara seized about three tons of tramadol. The trade in Tramadol also serves as a source of finance for terrorist groups such as Boko Haram or the Islamic State .
See also
Web links
Individual evidence
- ^ A b c The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; ISBN 978-0-911910-00-1 .
- ↑ a b Datasheet Tramadol hydrochloride from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ Entry on tramadol in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 31, 2018 or earlier.
- ^ A b c Römpp Lexikon Chemie, Georg Thieme Verlag, Stuttgart - New York, 10th edition, 1999, p. 4601.
- ^ Franz Gerstheimer: The unforeseen success of the pain reliever TRAMAL. Worldwide impact from the research laboratories. In: the scales. Magazine of Grünenthal GmbH, Aachen. Volume 35, 1996, No. 2, pp. 72-80.
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2nd, revised and expanded edition. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 2005, ISBN 3-8047-2113-3 , p. 133 .
- ↑ Hans Walter Striebel: Therapy of Chronic Pain: A Practical Guide . Schattauer Verlag, 4th edition 2001, ISBN 978-3-7945-2146-3 , p. 24.
- ↑ Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL (1992): Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an 'atypical' opioid analgesic. J Pharmacol Exp Ther 260: 275-285.
- ↑ a b Bernd Schäfer: Natural substances of the chemical industry, Elsevier, 2007, pp. 255, 256, ISBN 978-3-8274-1614-8 .
- ↑ Eberhard Klaschik : Pain therapy and symptom control in palliative medicine. In: Stein Husebø , Eberhard Klaschik (ed.): Palliative medicine. 5th edition, Springer, Heidelberg 2009, ISBN 3-642-01548-4 , pp. 207-313, here: p. 234.
- ↑ Safarinejad, Mohammad, Reza MD; Hosseini, Seyyed Yoosof MD: Safety and Efficacy of Tramadol in the Treatment of Premature Ejaculation: A Double-blind, Placebo-Controlled, Fixed-Dose, Randomized Study. In: Journal of Clinical Psychopharmacology , 2006, 26: 27-31.
- ↑ Salem, EA, Wilson, SK, Bissada, NK, Delk, JR, Hellstrom, WJ and Cleves, MA (2008): Tramadol HCL has Promise in On-Demand Use to Treat Premature Ejaculation. In: The Journal of Sexual Medicine , 5: 188-193, doi : 10.1111 / j.1743-6109.2006.00424.x .
- ↑ Sample technical information from the BfArM on tramadol hydrochloride . As of June 2016.
- ↑ Tramadol drops not for children under the age of 12 years .
- ↑ https://www.medsafe.govt.nz/safety/EWS/2018/Tramadol.asp Tramadol Safety Communication
- ↑ HYPERACUSIS UNDER TRAMADOL (TRAMAL, GENERICS). Retrieved July 3, 2020 .
- ↑ LS Nelson, D. Juurlink: Tramadol and Hypoglycemia: One More Thing to Worry About . In: JAMA Internal Medicine, 2015 , 175 (2) , pp. 194-195, doi : 10.1001 / jamainternmed.2014.5260
- ↑ JP Fournier, H. Yin, J.-L Montastruc, L. Azoulay: Tramadol for Noncancer Pain and the Risk of Hyponatremia . In: The American Journal of Medicine, 2015 , 128 (4) , pp. 418-425 doi : 10.1016 / j.amjmed.2014.10.046
- ↑ Yılmaz B, Erdem AF: Simultaneous Determination of Tramadol and its Metabolite in Human Plasma by GC / MS. , J AOAC Int. 2015 Jan-Feb; 98 (1): 56-61, PMID 25857879
- ↑ Yu H, Hong S, Jeong CH, Bae JW, Lee S: Development of a linear dual column HPLC-MS / MS method and clinical genetic evaluation for tramadol and its phase I and II metabolites in oral fluid. , Arch Pharm Res. 2018 Mar; 41 (3): 288-298, PMID 29196917
- ↑ Yilmaz B, Erdem AF: Simultaneous Determination of Tramadol and Its Metabolite in Human Urine by the Gas Chromatography-Mass Spectrometry Method. , J Chromatogr Sci. 2015 Aug; 53 (7): 1037-43, PMID 25616987
- ↑ Verri P, Rustichelli C, Palazzoli F, Vandelli D, Marchesi F, Ferrari A, Licata M: Tramadol chronic abuse: an evidence from hair analysis by LC tandem MS. , J Pharm Biomed Anal. 2015 Jan; 102: 450-8, PMID 25459945
- ↑ Scher ML, et al .: Potential interaction between tramadol and warfarin . In: Ann Pharmacother 1997; 31: 646-647.
- ↑ Woggon, Brigitte (2005) Treatment with psychotropic drugs (2nd edition). Bern: Hans Huber. ISBN 3-456-83538-8 .
- ↑ Serotonin syndrome under the analgesic Tramadol (Tramal et al.) , Arznei-telegramm (1/2002)
- ↑ Abanmy NO, et al. Compatibility of tramadol hydrochloride injection with selected drugs and solutions . In: Am J Health Syst Pharm 2005; 62: 1299-1302.
- ^ A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications , 4th edition (2001) Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 , pp. 2085.
- ↑ Zynovy Itov and Harold Meckler: A Practical Procedure for the Resolution of (+) - and (-) - tramadol . In: Organic Process Research & Development 2000 , 291-294.
- ↑ D. Burke and DJ Henderson: Chirality: a blueprint for the future , British Journal of Anaesthesia 88 (2002), 563-576.
- ↑ (a) K. Flick and E. v. Frankus, U.S. Patent 3,652,589 (Grünenthal GmbH) March 28, 1972; Chemical Abstracts 76 (1972) 153321. (b) K. Flick and E. Frankus, U.S. Patent 3,830,934 (Grünenthal GmbH) August 20, 1974; Chemical Abstracts 82 (1974) 21817.
- ↑ Examples: E. v. Frankus, E. Friedrichs, SM Kim and G. Osterloh, Arzneimittel-Forschung / Drug Research 28 (1978), 114-121. (b) K. Flick, E. v. Frankus and E. Friedrichs, Arzneimittel-Forschung / Drug Research 28 (1978), 107-113. (c) YA Ardakani and M.-R. Rouini, Journal of Pharmaceutical and Biomedical Analysis 44 (2007) 1168-1173.
- ↑ Physicians Desk Reference; Medical Economics Data, Oradell, NJ, 54 (2000), 2218-2219.
- ↑ Boumendjel, A; Sotoing Taïwe, G; Ngo Bum, E; Chabrol, T; Beney, C; Sinniger, V; Haudecoeur, R; Marcourt, L; Challal, S; Ferreira Queiroz, E; Souard, F; Le Borgne, M; Lomberget, T; Depaulis, A; Lavaud, C; Robins, R; Wolfender, JL; Bonaz, B; De Waard, M: Occurrence of the Synthetic Analgesic Tramadol in an African Medicinal Plant . In: Angewandte Chemie International Edition . 52, No. 45, November 2013, pp. 11780-11784. doi : 10.1002 / anie.201305697 .
- ↑ Souvik Kusari, Simplice Joel N. Tatsimo, Sebastian Zühlke, Ferdinand M. Talontsi, Simeon Fogue Kouam, Michael Spiteller: Tramadol A True Natural Product? . In: Angewandte Chemie International Edition . September 2014. doi : 10.1002 / anie.201406639 .
- ↑ Laura Salm-Reifferscheidt: West Africa is inundated by opioids , in: Deutschlandfunk Kultur from April 22, 2018, accessed on April 22, 2018.