Bromine isole
| Bromine isole | ||||||
| Surname | 2-bromoanisole | 3-bromoanisole | 4-bromoanisole | |||
| other names | o -Bromanisole | m -bromanisole | p -bromanisole | |||
| Structural formula |

|

|

|
|||
| CAS number | 578-57-4 | 2398-37-0 | 104-92-7 | |||
| PubChem | 11358 | 16971 | 7730 | |||
| Molecular formula | C 7 H 7 BrO | |||||
| Molar mass | 187.04 g mol −1 | |||||
| Physical state | liquid | |||||
| Melting point | 2-3 ° C | 2 ° C | 9-10 ° C | |||
| boiling point | 223 ° C | 210-211 ° C | 223 ° C | |||
| Refractive index | 1.5727 (20 ° C; 589 nm) | 1.5635 (20 ° C; 589 nm) | 1.5642 (20 ° C; 589 nm) | |||
|
GHS labeling |
|
|
|
|||
| H and P phrases | no H-phrases | no H-phrases | no H-phrases | |||
| no EUH phrases | no EUH phrases | no EUH phrases | ||||
| no P-phrases | no P-phrases | no P-phrases | ||||
In chemistry , the bromosoles form a group of substances that are derived from both anisole and bromobenzene . The structure consists of a benzene ring with attached methoxy group (–OCH 3 ) and bromine (–Br) as substituents . Their different arrangements ( ortho , meta or para ) result in three constitutional isomers with the empirical formula C 7 H 7 BrO.
presentation
Bromanisols can be prepared from the bromophenols by etherification with dimethyl sulfate .
4-Bromanisole is obtained from anisole by bromination with elemental bromine.
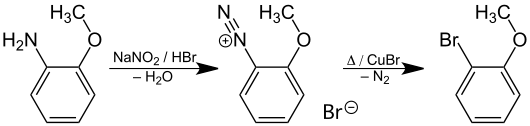
The Sandmeyer reaction starting from 2-methoxyaniline yields 2-bromanisole.
Individual evidence
- ↑ a b c data sheet 2-bromoanisole from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ Alfa Aesar Catalog 2008/09, p. 285.
- ↑ a b c Data sheet 4-Bromoanisole from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ a b Data sheet 3-Bromoanisole from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ a b c David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-56.
- ↑ Heinz GO Becker u. a .: Organikum . 19th edition. Barth, Leipzig 1993, ISBN 3-335-00343-8 , pp. 331-332.