Sandmeyer reaction
By means of a Sandmeyer reaction , named after its discoverer Traugott Sandmeyer (1854–1922), the amino group of an aromatic compound is substituted by a nucleophile by means of diazotization . The reaction is well suited for the chlorination and bromination of aromatics and is used when the substituent , such as bromine , for example , cannot be introduced into the aromatic nucleus by simply boiling off the diazonium salt. Also nitriles and aryl can be represented by a Sandmeyer reaction.
For fluorination , it is ill-suited, so instead the Schiemann reaction is used. In contrast, iodination takes place via a Sandmeyer-like reaction (see below).
The Rosenmund-von Braun reaction for the production of aryl nitriles is similar to the Sandmeyer reaction.
reaction
The aromatic amine is mixed with nitrite and the nucleophile (→ X) in the cold . The conversion takes place with the aid of the corresponding copper (I) salts as catalysts . The following reaction scheme shows the reaction of the diazonium ion with chloride as a nucleophile:
mechanism
The mechanism of this reaction after diazotization is not yet fully understood. It is assumed that the resulting diazonium compound is reduced from monovalent to divalent copper with oxidation (SET = "single electron transfer"). With elimination of N 2 , a is aryl - radical , which with the anion (Cl - , Br - , CN - ) connects the copper salt as the nucleophile. The Cu (II) is reduced to Cu (I) again via a further one-electron transfer, so it is a real catalyst.
The formation of biphenyl as a by-product from a dimerization makes a free radical reaction mechanism plausible. Other by-products can also be phenols , diaryls and azo compounds . The copper ion only serves as an electron acceptor or donor. However, alternative representations of the mechanism assume the copper (II) salt is bound to the aryl radical and then halide transfer from the copper.
Sandmeyer analogous reaction
Even if this reaction is not among the Sandmeyer reactions, aryldiazonium salts formed with sodium nitrite in an analogous manner nitroaromatics . The copper (I) salt is formed in situ from a copper (II) salt through a redox reaction with sodium nitrite. The nitrite thus serves both as a nucleophile and as a reducing agent.
Sandmeyer-like reaction
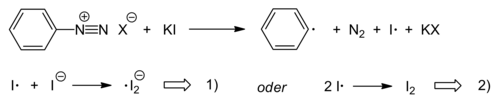
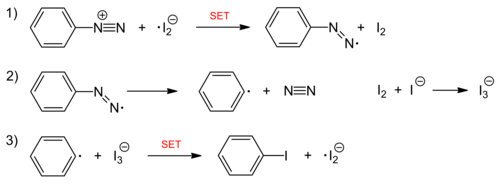
The iodination of aromatics is also possible by reacting aryldiazonium salts with potassium iodide . The nucleophile iodide itself and the resulting iodine species take over the function of the otherwise used copper (I) catalyst. The reaction is a radical chain reaction , the chain carrier of which is an iodine radical anion . The reaction is initiated with the reduction of the diazonium ion by iodide.
Start reaction:
Chain reaction:
Individual evidence
- ↑ Thomas Laue, Andreas Plagens: Name and catchword reactions of organic chemistry . Vieweg + Teubner, Wiesbaden 2009, ISBN 978-3-8351-0091-6 .
Web links
- Portal for Organic Chemistry Further information on the Sandmeyer reaction
- Sandmeyer reaction - who is behind it? ( Memento of March 10, 2007 in the Internet Archive )