Rosenmund-von-Braun reaction
The Rosenmund-von-Braun reaction is a name reaction in organic chemistry and is used to produce aryl nitriles from aryl halides . It was discovered by Karl Wilhelm Rosenmund in 1919 and modified by Julius von Braun in 1931 .
Overview reaction
The production of aryl nitriles (e.g. benzonitrile ) from aryl halides (e.g. phenyl bromide ) takes place with the addition of an excess of copper (I) cyanide in a polar, high-boiling solvent (e.g. pyridine , dimethylformamide (DMF), Nitrobenzene ) under reflux:
The Rosenmund-von-Braun reaction is a nucleophilic aromatic substitution reaction and is related to the Sandmeyer reaction , which takes place under radical conditions. It is also similar to the Stephens-Castro coupling .
mechanism
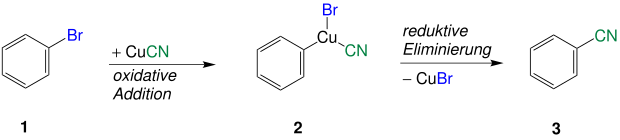
A possible mechanism - explained here using the example of phenyl bromide - can be understood through the formation of a Cu (III) species. It proceeds according to a two-step addition-elimination mechanism, as is often the case with nucleophilic aromatic substitutions. The first step involves the oxidative addition of the phenyl bromide ( 1 ) with the catalytically active Cu (I) species in the copper cyanide. The resulting Cu (III) intermediate 2 reacts by reductive elimination to form phenyl cyanide ( 3 ) and copper bromide.
Web links
- Rosenmund-von Braun reaction in the portal for organic chemistry
Individual evidence
- ↑ S. Budavari, MN Finish LY Stroumtsos, M. Wind Wood: The Merck Index - An Encyclopedia of Chemicals and Drugs . 9th edition. Merck & Co., 1976, ISBN 0-911910-26-3 , pp. 54-56 .
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, 2009, ISBN 978-0-471-70450-8 , pp. 2425-2428 .
- ↑ R. Brückner : reaction mechanisms, organic reactions, stereochemistry, modern synthesis methods . 3. Edition. Springer, Munich 2004, ISBN 3-8274-1579-9 , pp. 692 .
- ^ RD Stephens, CE Castro: The Substitution of Aryl Iodides with Cuprous Acetylides. A Synthesis of Tolanes and Heterocyclics. In: J. Org. Chem. 28, No. 12, 1963, pp. 3313-3315, doi: 10.1021 / jo01047a008
- ↑ DC Owsley and CE Castro: Substitution of Aryl Halides with Copper (I) Acetylides: 2-Phenylfuro [3,2-b] Pyridine In: Organic Syntheses . 52, 1972, p. 128, doi : 10.15227 / orgsyn.052.0128 ; Coll. Vol. 6, 1988, p. 916 ( PDF ).
- ↑ H.-J. Cristau, A. Ouali, J.-F. Spindler, M. Taillefer: Mild and Efficient Copper-Catalyzed Cyanation of Aryl Iodides and Bromides In: Chem. Eur. J. No. 11, 2005, pp. 2483-2492, doi: 10.1002 / chem.200400979 .
- ^ PP Cellier, H.-J. Cristau, J.-F. Spindler, M. Taillefer: Highly Efficient and Mild Copper-Catalyzed N- and C-Arylations with Aryl Bromides and Iodides In: Chem. Eur. J. 22, No. 10, 2004, pp. 5607–5622, doi: 10.1002 / chem . 200400582 .