Benzonitrile
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
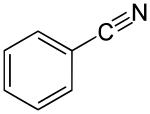
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Benzonitrile | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 5 N | |||||||||||||||
| Brief description |
colorless liquid with a pleasant smell of bitter almond |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 103.12 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.01 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−13 ° C |
|||||||||||||||
| boiling point |
191 ° C |
|||||||||||||||
| Vapor pressure |
72 Pa (20 ° C) |
|||||||||||||||
| solubility |
heavy in water (10 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.5289 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Benzonitrile is a colorless, flammable liquid that smells like bitter almonds.
The molecule consists of a six-membered aromatic ring to which a nitrile group is attached. It is used as a chemical starting material for syntheses or very rarely as a solvent .
use
The benzylamine is produced by catalytic hydrogenation (reduction) of benzonitrile . However, if benzonitrile is mixed with strong aqueous acids or bases , it hydrolyzes via benzamide to benzoic acid and ammonia .
safety instructions
When burned, gases containing hydrocyanic acid can be produced.
Individual evidence
- ↑ Entry on benzonitrile. In: Römpp Online . Georg Thieme Verlag, accessed on November 12, 2014.
- ↑ a b c d e f g h Entry on benzonitrile in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-38.
- ↑ Entry on Benzonitrile in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ Heinz GO Becker, Werner Berger, and Günter Domschke: Organikum . 22nd edition. Wiley-VCH, 2004, ISBN 978-3-527-31148-4 .
