Benzylamine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
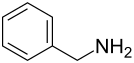
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Benzylamine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 7 H 9 N | |||||||||||||||||||||
| Brief description |
colorless, air-smoking liquid with an ammonia-like odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 107.15 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.983 g cm −3 |
|||||||||||||||||||||
| Melting point |
10 ° C |
|||||||||||||||||||||
| boiling point |
185 ° C |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| pK s value |
pK b = 4.65 |
|||||||||||||||||||||
| solubility |
Miscible in all proportions with ethanol and diethyl ether, 3.1 g · l −1 in water, it dissolves with a strongly alkaline reaction |
|||||||||||||||||||||
| Refractive index |
1.5401 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Benzylamine is a chemical compound that consists of a benzyl group and an amino group attached to it . It is the simplest aromatic amine with the amino group in the side chain.
properties
Benzylamine is a colorless, hardly flammable, air-smoking liquid with an ammonia-like odor, which is completely miscible with water. It decomposes above 300 ° C, with toluene , benzonitrile , stilbene , bibenzyl , ammonia , nitrogen oxides , carbon monoxide and carbon dioxide being formed.
Safety-related parameters
Benzylamine forms flammable vapor-air mixtures. The compound has a flash point of 65 ° C. The explosion range is between 0.7% by volume as the lower explosion limit (LEL) and 8.2% by volume as the upper explosion limit (UEL). The ignition temperature is 390 ° C. The substance therefore falls into temperature class T2.
presentation
There are various syntheses for benzylamine:
- Reaction of benzyl chloride with ammonia
- Reaction of N -benzylphthalimide with hydrazine according to the principle of the Gabriel synthesis
- from benzyl bromide and acetamide and subsequent cleavage of the amide bond
- Reduction (hydrogenation) of benzamide or benzonitrile with lithium aluminum hydride
use
It is a frequently used starting compound in synthetic organic chemistry.
Individual evidence
- ↑ a b c d e f g h i j k l m Entry on benzylamine in the GESTIS substance database of the IFA , accessed on August 20, 2018(JavaScript required) .
- ↑ a b Brockhaus ABC Chemie, VEB FA Brockhaus Verlag Leipzig 1965, p. 172.
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Entry on Benzylamines in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ a b c d The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals , 14th Edition, 2006, ISBN 978-0-911910-00-1 , p. 186.

