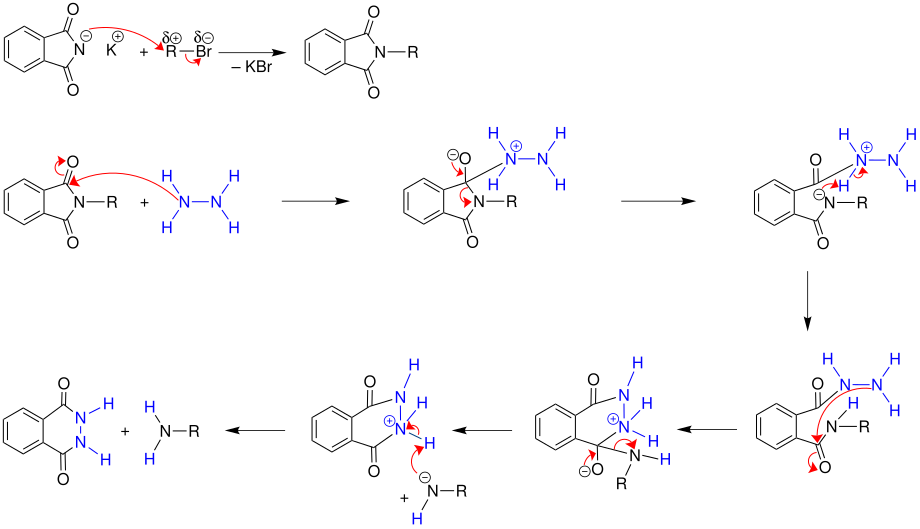
Gabriel synthesis
The Gabriel synthesis is a name reaction of organic chemistry , which was named after its discoverer, the German chemist Siegmund Gabriel (1851-1924). It is a chemical method for the selective production of primary amines by hydrolysis or hydrazinolysis of phthalimides . A historically interesting variant of this synthesis is the synthetic preparation of α- amino acids that took place in 1889 .
Overview reaction
Potassium phthalimide reacts with a haloalkane to form the corresponding N- alkyl phthalimide, which reacts in several reaction steps by hydrolysis or hydrazinolysis to form a primary amine .
Reaction mechanism
Hydrazinolysis
In the first step, potassium phthalimide reacts with a haloalkane (here an alkyl bromide) to form the corresponding N -alkyl phthalimide. In the next step, in the case of milder and better hydrazinolysis, the N- alkylphthalimide formed is reacted with hydrazine. The hydrazinolysis takes a few hours in boiling ethanol . Under these mild conditions, no side reactions usually occur. The products of this so-called Ing-Manske variant are a cyclic phthalhydrazide and the desired primary amine. Instead of hydrazinolysis, the amine synthesis can also take place by alkaline hydrolysis. However, the reaction proceeds only very slowly or under drastic conditions. The hydrazinolysis proceeds so well because of the neighboring lone pairs of electrons in the hydrazine, because the nucleophilicity of the hydrazine is so pronounced because of the ( α-effect ).
hydrolysis
In addition to hydrazinolysis, the alkaline hydrolysis of the alkyl phthalimides is an alternative method for the production of primary amines. The N -alkylphthalimide is converted into a phthalic acid anion and an alkylamine by means of highly concentrated sodium hydroxide solution .
Reaction with bromomalonic acid ester
If, instead of haloalkanes bromine malonic acid ester used as the substrate, α- are amino acids accessible. The first stage of the reaction proceeds as described above, except that the alkyl radical R is replaced by the malonic acid ester. The following figure shows the further course of the reaction ( hydrolysis ) from the amine malonic acid ester to the α-amino acid glycine .
The N -Phthalimidomalonsäureester can be a variety of alkyl halides or α, β- unsaturated carbonyl compounds alkylated be so different α-amino acids can be represented.
Practical meaning
The Gabriel synthesis had to be developed because the synthesis of primary amines from haloalkanes and ammonia is not possible. The intermediate primary amine, as a better nucleophile, reacts much faster with the haloalkane than it is formed from ammonia. The consequence of this is that only very small amounts of primary amine can be isolated from the reaction mixture at the end. The main products of this reaction would be the tertiary amine and the quaternary ammonium salt.
The Gabriel synthesis is a purely laboratory process. Because of the formation of stoichiometric amounts of several waste materials , the atom economy of the Gabriel synthesis is so bad that nobody realizes a technical synthesis for primary amines based on this reaction.
Another way of preparing primary amines with higher atom economy is the reaction of the haloalkane with sodium azide to form the alkyl azide , from which the primary amine can then be obtained reductively (e.g. with lithium aluminum hydride ). The reaction of the haloalkane with sodium cyanide with subsequent reduction to the primary amine proceeds with chain extension .
Individual evidence
- ^ LF Fieser, M. Fieser , Textbook of Organic Chemistry, Verlag Chemie, 3rd edition, 1957.
- ↑ L. Kürti, B. Czakó: Strategic Applications Of Named Reactions In Organic Synthesis . Elsevier Academic Press, USA 2005, pp. 182 .
- ↑ T. Laue, A. Plagens: Name and catchword reactions of organic chemistry . Teubner Verlag, 2006, ISBN 3-8351-0091-2 , p. 146-149 .
literature
- T. Laue, A. Plagens: Name and catchword reactions in organic chemistry. (= Teubner Chemistry Study Books ). 3. Edition. Stuttgart 1998, ISBN 3-519-23526-9 .