Phthalimide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phthalimide | |||||||||||||||
| other names |
1,2-benzene dicarboximide |
|||||||||||||||
| Molecular formula | C 8 H 5 NO 2 | |||||||||||||||
| Brief description |
white, crystalline powder or flakes |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 147.13 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.21 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
238 ° C |
|||||||||||||||
| boiling point |
310 ° C |
|||||||||||||||
| pK s value |
8.3 |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
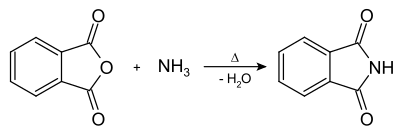
Phthalimide is a chemical compound with the molecular formula C 8 H 5 NO 2 . The white, crystalline compound is the imide of phthalic acid .
Extraction and presentation
The phthalic acid imide is synthesized by reacting phthalic anhydride with ammonia .
properties
Under normal conditions, phthalimide is an air-insensitive solid that can oxidize under the action of light. Due to the electron-withdrawing (–M) effect of the two carbonyl groups, phthalimide has a pronounced NH acidity . The flash point is 150 ° C, the ignition temperature is> 500 ° C.
Usage and reactions
The imide group has an acidic reaction (pK a = 8.3) and can easily be converted to potassium phthalimide with alcoholic potassium hydroxide solution .
Potassium phthalimide is a reagent for the synthesis of primary amines according to the principle of the Gabriel synthesis . Phthalimide is also required in pesticides (e.g. folpet ), dyes and as a raw material for anthranilic acid .
Individual evidence
- ↑ a b c d Entry on phthalimide. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ a b c d e f Entry on phthalimide in the GESTIS substance database of the IFA , accessed on December 20, 2019(JavaScript required) .
- ^ A b c Hans Beyer and Wolfgang Walter : Organische Chemie , S. Hirzel Verlag, Stuttgart, 1984, page 521, ISBN 3-7776-0406-2 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 490, ISBN 3-342-00280-8 .