Potassium phthalimide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium phthalimide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 4 KNO 2 | |||||||||||||||
| Brief description |
light yellow, odorless powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 185.2 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.63 g cm −3 |
|||||||||||||||
| Melting point |
> 300 ° C (decomposition) |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Potassium phthalimide is the potassium salt of phthalimide .
Extraction and presentation
The reaction of phthalimide with alcoholic potassium hydroxide solution produces potassium phthalimide.
properties
Potassium phthalimide is a flammable light yellow solid that is soluble in water. It decomposes above 300 ° C, producing nitrogen oxides , carbon monoxide and carbon dioxide .
Usage and reactions
Potassium phthalimide is a reagent for the synthesis of primary amines according to the principle of the Gabriel synthesis .
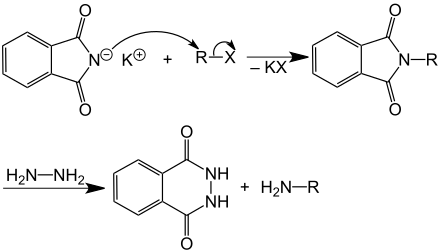 Use of potassium phthalimide in the Gabriel synthesis of a primary amine. R is an organyl radical, X is a halogen atom, e.g. B. chlorine or bromine.
Use of potassium phthalimide in the Gabriel synthesis of a primary amine. R is an organyl radical, X is a halogen atom, e.g. B. chlorine or bromine.
Phthalimide is also required for the synthesis of pesticides (e.g. Folpet ), dyes and anthranilic acid .
Individual evidence
- ↑ a b c d e f Entry for CAS no. 1074-82-4 in the GESTIS substance database of the IFA , accessed on July 18, 2011(JavaScript required) .
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 1072.