Nucleophilic aromatic substitution
In organic chemistry, nucleophilic aromatic substitution refers to a number of different nucleophilic substitution reactions on aromatics . This type of substitution is formally one of the substitutions on the unsaturated carbon atom. Based on the abbreviation for nucleophilic substitution, this mechanism is also referred to as S N Ar (aromatic). The nucleophilic substitution generally is discussed in more detail in a separate article.
General
Nucleophilic reagents (i.e. reagents that themselves have a negative charge or a free electron pair) look for locations in molecules with an electron deficit (= locations with positive charges / partial charges or electron gaps). “Normal” aromatics with their six π electrons are naturally not very reactive to them. Therefore, depending on the mechanism, there are different requirements for a nucleophilic substitution to take place.
Mechanisms
An S N 2 -like reaction, as in the case of aliphatic nucleophilic substitution, is not possible on the aromatic because the configuration of a substituent on the aromatic cannot be inverted . The attack would have to come from inside the benzene ring and twist it in an impossible way.
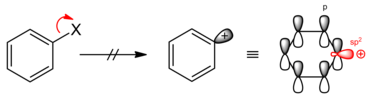
An S N 1-like reaction is also not possible, since the aryl cations formed in this case, in particular the phenyl cation, are not stable due to the positive charge (electron gap ) located in the sp 2 orbital . Because of its localization, the charge cannot be stabilized by mesomerism .
An exception are the aryldiazonium salts , wherein the N 2 -Freisetzung serves as energy compensation. The release and escape of nitrogen are the driving force behind this reaction.
The nucleophilic aromatic substitution cannot therefore take place via a one-step mechanism. In general, there are four different substitution mechanisms:
- Addition-Elimination Mechanism ( S N Ar )
- Elimination-Addition Mechanism ( Arine Mechanism )
- Substitution on aryldiazonium ions
- via aryl cations ( S N 1 ), see phenol boiling , Schiemann reaction
- Free radical or electron transfer processes, e.g. B. the Sandmeyer reaction
-
Radical nucleophilic aromatic substitution
- starting with a reduction ( S RN 1 )
- starting with an oxidation ( S ON 2 )
Addition-Elimination Mechanism
While with electrophilic aromatic substitution usually a hydrogen (but see also ipso-substitution ) is exchanged for an electrophile , the substitution of this is not possible with a nucleophilic aromatic substitution. The hydrogen would have to leave the molecular assembly in the form of the extremely poor leaving group hydride (= H - ).
The prerequisites for nucleophilic aromatic substitution are therefore
- the electron density in the aromatic must be reduced by (−I / −M) substituents.
- the aromatic must have a good leaving group that is substituted.

The attacking nucleophilic group attacks the substituted carbon atom. The nucleofugal leaving group emerges from the molecular assembly, taking the former binding electrons with it. An intermediate stage is formed at which an additional negative charge occurs in the aromatic. This negative charge is delocalized over the entire ring and over the (−M) substituent.
The (-) - M substituent therefore enables the nucleophile to attack and also stabilizes the intermediate stage.
Vicarious S N Ar
The substitute S N Ar or Vicarious S N Ar represents a special case of the addition-elimination mechanism. The nucleofug is brought along directly in the nucleophile. As a result, a hydrogen atom on the aromatic can also be substituted indirectly.
Transitional or Intermediate?
The reaction of 2-ethoxy-1,3,5-trinitrobenzene (ethyl picric acid) with sodium methoxide yields a salt isolated by Jakob Meisenheimer for the first time. The isolable salts of the intermediates in nucleophilic aromatic substitution are known as Meisenheimer complexes .
They are considered to be evidence that nucleophilic aromatic substitutions occur in a two-step mechanism (addition-elimination mechanism).
For electrophilic aromatic substitutions , George Willard Wheland found corresponding Wheland intermediates or Wheland complexes named after him .
Known uses
Sanger's reagent , used as a tool for sequence analysis in peptides; see Sanger's method for determining the amino acid sequence
literature
- Peter Sykes: How do organic reactions work? , 2nd edition, Wiley-VCH 2001, ISBN 3-527-30305-7
- Peter Sykes: reaction mechanisms of organic chemistry , 7th edition, Verlag Chemie 1979, ISBN 3-527-21047-4
- Author collective: Organikum , 22nd edition, Wiley-VCH, 2004, pp. 393-399, ISBN 978-3-527-31148-4