Arine
| Didehydrobenzene as a diradical ( empirical formula: C 6 H 4 ) |
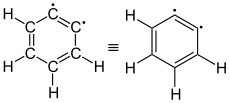 ortho -Didehydrobenzol (1,2-benzyne) |
 meta -idehydrobenzene (1,3-didehydrobenzene) |
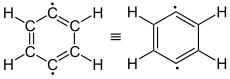 para -idehydrobenzene (1,4-didehydrobenzene) |
In chemistry , arynes are uncharged, short-lived, reactive and cyclic intermediates . They are derived from aromatics , whereby two adjacent substituents, mostly in ortho , have been split off, resulting in two atomic orbitals with two electrons distributed between these two carbon atoms . Formally, ortho- didehydro-arynes have a carbon-carbon triple bond at this point . Due to the tensioned triple bond, such arynes have energetically very deep-lying LUMOs . Therefore arynes are correspondingly reactive and tend to undergo addition reactions . In analogy to carbenes and nitrenes , arynes have a singlet and a triplet state .
history
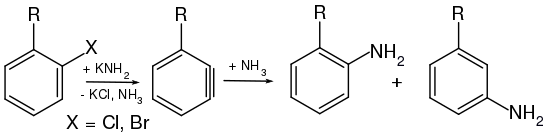
As early as 1902, Richard Stoermer and Bruno Kahlert , who worked at the University of Rostock, postulated that the formation of 2-ethoxybenzofuran from the reaction of 3-bromobenzofuran with bases in ethanol takes place via the reactive intermediate stage of 2,3-didehydrobenzofuran. WE Bachmann and HT Clarke also suspected didehydrobenzene as a reactive intermediate in the Wurtz-Fittig synthesis in 1927 . Further investigations were carried out in 1953 by John Dombrowski Roberts , who was able to prove the existence of arynes through the reaction of 14 C-labeled chlorobenzene with potassium amide and subsequent reaction with ammonia . Roberts discovered that the newly introduced NH 2 group was located on both the 14 C carbon atom and the adjacent carbon atom ( cine substitution ). The formation of both products in equal amounts can only be explained by the existence of the symmetrical intermediate, since the classic nucleophilic aromatic substitution , which proceeds via an addition-elimination route, only provides the isomer in which the NH 2 group is attached to the 14 C- Atom is bound.
In 1960 Rolf Huisgen and Jürgen Sauer confirmed these results; they found an isomer ratio of 48 percent to 52 percent, which can be attributed to the isotopic effect . Erwin. F. Jenny and John D. Roberts found similar results when reacting 14 C-labeled fluorobenzene with phenyllithium . After hydrolysis, they obtained a biphenyl with a rearrangement rate of 53 percent.
In 1956 Georg Wittig succeeded in demonstrating the reactive aryne intermediates by trapping reactions with furan in various Diels-Alder reactions . IP Fisher and FP Lossing found the peak of dehydrobenzene in mass spectrometric studies in 1963 and were able to determine its ionization potential . In 1969 the first mass spectroscopic detection of 9,10-dehydrophenanthrene was achieved by Hans-Friedrich Grützmacher and Joachim Lohmann . The first infrared spectroscopic detection of benzyne succeeded OL Chapman by photolysis of phthaloyl peroxide , Benzocyclobutadienon or phthalic anhydride and matrix isolation spectroscopy at low temperatures. Chapman wrongly assigned a band at 2085 cm −1 to the stretching vibration of the CC triple bond , which was later confirmed by other research groups. It was not until 1992 that Juliusz G. Radziszewski finally succeeded in proving that the stretching vibration of the CC triple bond produces a band at 1846 cm −1 . This corresponds to the expectation that the (formal) CC triple bond due to the ring strain is weaker than that in unstrained alkynes, which show a band around 2150 cm −1 . By trapping reactions with furan in a Diels-Alder reaction, Hans F. Ebel and Reinhard W. Hoffmann were able to calculate the lifetime of didehydrobenzene in a vacuum to a maximum of 20 ms . Ralf Warmuth finally succeeded in isolating 1,2-didehydrobenzene in a molecular container and investigating 1 H and 13 C NMR spectroscopy. By comparison with benzene, the NMR data could be calculated as follows: 1 H-NMR δ (D 8 THF) = 7.69 ppm and 7.01 ppm; 13 C-NMR δ (D 8 THF) = 182.7 ppm, 126.8 ppm and 138.2 ppm.
The discovery of the aryne intermediate
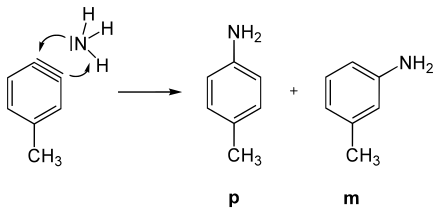
The postulate of an aryne intermediate was made on the basis of a series of experiments with chlorobenzene (C 6 H 5 Cl). Chlorobenzene, a rather electron-rich aromatic ( + M effect of chlorine), proves to be very inert towards most nucleophiles such as the hydroxide ion (OH - ); temperatures of over 200 ° C are necessary to generate a reaction at all. With sodium amide (NaNH 2 ) in liquid ammonia , however, chlorobenzene is converted into aniline at −33 ° C.
If the starting compound is not considered to be chlorobenzene but rather p -chlorotoluene ( p -CH 3 C 6 H 4 Cl) and it is reacted with amide ions under the same conditions as for chlorobenzene, two products are found: an expected amino substitution product and an unexpected one. Thus, the chlorine atom cannot have been substituted directly by the amino group . In addition, when chlorotoluene is reacted with amide ions, only the meta and para amino products are found, never the ortho isomer.
Reaction mechanism
It is known from H / D exchange reactions that the amide anion in liquid ammonia can abstract protons from benzene. This goes particularly quickly in the o -position to (−I) -substituents, such as B. chlorine. From these facts it can be concluded that primarily the chlorine-substituted carbon atom of the aromatic is not attacked. As a strong base, the amide ion deprotonates an alpha hydrogen atom in chlorotoluene, which leads via a carbanion to a triple bond intermediate, the aryne.
In the subsequent addition of NH 3 , both the m -isomer and the p -isomer are formed.
2,6 – Dimethylchlorobenzene does not react to form the corresponding aniline derivative under these conditions. 2,6 – Dimethylchlorobenzene has no α-hydrogen atoms. Thus no proton can be abstracted, the aryne does not form.
Didehydrobenzene

The “additional” π bond is marked in blue .
The simplest aryne, C 6 H 4 ( labeled 1 in the right picture), is sometimes called benzyne based on the English name . However, this designation must be viewed critically, as it implies a special triple bond. A better name is didehydrobenzene or didehydrobenzene , usually referred to as dehydrobenzene for short . Dehydrobenzene is resonance stabilized , as structures 1 and 2 show. The actual distribution of the electrons is from 3 more apparent. The additional π bond is located in 4a and is perpendicular to the π bonds (in 4b ) of the aromatic system. Dehydrobenzene can be described as a diradical : The “additional” π bond in 2 , 3 , 4a and 4b is then split homolytically , with one electron each standing next to the atom that was previously involved in the “additional” π bond.
Due to the triple bond, dehydrobenzene is extremely reactive and therefore has a very short lifespan. In solutions it reacts very quickly with existing reactants and in the gas phase with itself to form di- or triphenylene . While in alkynes (in the simplest case ethyne ) the unhybridized p orbitals are usually orthogonal up and back to the molecular axis, which results in an optimal overlap of the orbitals, in aryne the p orbital is distorted in order to accommodate the triple bond in the ring system. This reduces the optimal overlap of the orbitals.
There are three possible diradicals of dehydrobenzene: 1,2-, 1,3- and 1,4-didehydrobenzene. The binding energies are in silico 106, 122, and 138 kcal / mol (444, 510, and 577 kJ / mol). Maitland Jones at Princeton investigated the possible rearrangements of 1,2-, 1,3- and 1,4-didehydrobenzenes.
ortho -idehydrobenzene
The ortho -Didehydrolbenzol is the best studied isomer from the group of the three possible Didehydrobenzenes. The illustration and its reactions will be dealt with accordingly in the following. As the large number of publications shows, this is certainly due to the very simple experimental accessibility through a large number of possible formation reactions, but also to the fact that the two electrons can pair and thus reach the energetically more favorable singlet state. Armin Schweig calculated the bond length for the CC triple bond in ortho -didehydrobenzene (depending on the calculation model) to be 122 to 126 pm. Anita M. Orendt determined a value of 124 ± 2 pm. This is a value that is close to a normal C≡C triple bond, such as ethyne (120.3 pm). By η -2 coordination of the C≡C triple bond in the metal complex, ortho- dihydrobenzenes and even tetradehydrobenzene can be stabilized and used specifically for catalyzed organic syntheses.
meta -idehydrobenzene
In contrast to the ortho- didehydroarines, the meta and para isomers are significantly more unstable and reactive. According succeeded only in 1992 working groups to Wolfram Sander and Dieter Cremer, the first IR spectroscopic proof of the existence of 2,4-Didehydrophenol by photolysis of matrix- quinonediazide at 10 K .
The first isolation of 1,3-didehydrobezole was achieved in 1996 in Wolfram Sander's group by photolysis of meta - para -cyclophane-9,10-dione or by gas-phase thermolysis of diacetyl peroxide . Other ways of preparing meta-didehydrobenzene are the pyrolysis of 1,3-diiodobenzene or flash vacuum pyrolysis of 1,3-dinitrobenzene . The 1,3-didehydrobenzene is not very stable under the chosen conditions and rearranges itself to 3-hexene-1,5-diyne through a ring opening reaction.
para -idehydrobenzene
In 1974 Richard Jones and Robert Bergman were able to show that at positions C-1 and C-6, deuterated ( Z ) -hex-3-ene-1,5-diyne was converted into a mixture of 3,4- and 1,6-deuterated ( Z ) -hex-3-en-1,5-diyne passes over, but no 1,3- or 1,4-deuterated isomers are formed. The result can only be explained by a symmetrical and cyclic intermediate, 1,4-didehydrobenzene.
The ring closure reaction is called the Bergman cyclization after its discoverer . The fact that 1,4-didehydrobenzene is actually formed could be demonstrated by appropriate trapping reactions, for example by the formation of 1,4-dichlorobenzene in the presence of carbon tetrachloride . The work of Kyriacos Costa Nicolaou has shown that the rate of Bergmann cyclization depends on how close the two terminal carbon atoms (C-1 and C-6) are. In ( Z ) -Hex-3-en-1,5-diyne, the two terminal carbon atoms are 412 pm apart , and the cyclization takes place at 200 ° C. with a half-life of 30 s . If the ends of the en-diine , e.g. B. brought closer together by a ring closure, the reaction rate increases. In 3-cyclodecen-1,5-diyne, the distance between C-1 and C-6 is only 325 pm, and the Bergman cyclization already takes place at room temperature with a half-life of 18 h . But the presence of substituents also has an influence on the rate of the Bergmann cyclization.
The Bergman cyclization is a reversible reaction in which 1,4-didehydrobenzene is converted back into ( Z ) -hex-3-ene-1,5-diyne by a retro- Bergmann reaction . Since en-diyne is the energetically more stable product compared to 1,4-didehydrobenzene, the direct detection and isolation of 1,4-didehydrobenzene is correspondingly difficult. The thermolysis of 1,4-diiodobenzene gives only hex-3-en-1,5-diyne, 1,4-didehydrobenzene cannot be detected spectroscopically. In a photolysis process developed by Juliusz G. Radziszewski, however, 1,4-didehydrobenzene could be represented and characterized in a neon matrix at 6 K from 1,4-diiodobenzene by irradiation at 248 nm .
Hetarine
Arynes in which a carbon atom has been formally replaced by a heteroatom are referred to as hetarines . The presentation and reactions of the hetarins have also been examined in numerous research projects. Like the arynes, hetarines are often formed via elimination reactions of halogenated heteroaromatic compounds. In their reactions, too, hetarines behave similarly to substituted arynes, whereby the heteroatom can have a stronger directing effect. The position of the hetero atom in the aromatic ring system plays a decisive role. A working group led by Thomas Kauffmann was able to show that with halogenated quinolines, chlorine and bromine in the 5-, 6- or 7-position are split off via arynes according to the elimination-addition mechanism, while 8-position chlorine, bromine or iodine are split off after the addition -Out of elimination is replaced. Well-known representatives of the hetarines are about 3,4-didehydropyridine, 3,4-didehydroquinoline or the above-mentioned 2,3-didehydrobenzofuran. In the case of halogen derivatives of pyridine, the formal formation of the triple bond can take place in the 2,3 or 3,4 position to the nitrogen atom.
presentation
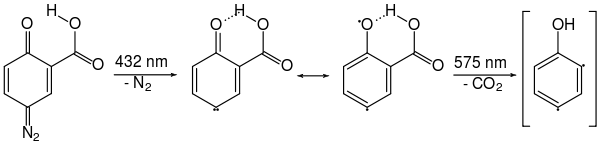
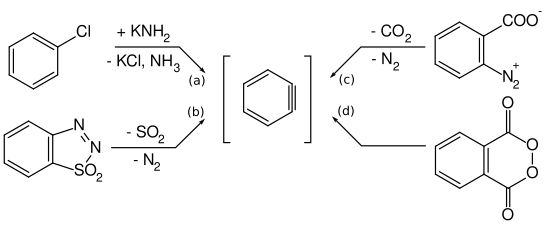
Arynes are mostly prepared from aryl halides by reaction with strong bases according to equation (a). But other reaction pathways have also been described. Georg Wittig and Reinhard W. Hoffmann investigated the formation of didehydrobenzene through the decomposition of benzothiadiazole oxide at room temperature according to equation (b). Martin Stiles and Roy G. Miller report a similar reaction path by thermolysis of benzene diazonium o -carboxylate in boiling furan according to equation (c). Georg Wittig and Hans F. Ebel also reported on the thermolysis of phthaloyl peroxide in a vacuum at 600 ° C. according to equation (d).
The thermolysis of Benzoldiazonioum- o -carboxylate, such as diazotized anthranilic acid , according to equation (c) takes place via a concerted mechanism:
Arynes can also be prepared by reacting 1,2-fluorobromobenzene with lithium, photolysis reactions or via Grignard compounds with aromatic radicals.
Investigations into the formation of arynes from aryl halides by reaction with phenyllithium in diethyl ether show that this takes place via a two-step mechanism. In the first step, an ortho-hydrogen atom, which is in the immediate vicinity of the halogen atom, is split off and replaced by an alkali metal . Then, in the second step, the aryne is formed by splitting off the lithium halide.
Rolf Huisgen was able to show that under appropriately chosen conditions (phenyllithium in diethyl ether ) the fluorobenzene reacts about 10 times faster than the other aryl halides (fluorine >> bromine> chlorine> iodine). Georg Wittig and Liselotte Pohmer were also able to show that the formation of dehydrobenzene is easier the more electronegative the halogen and the more electropositive the metal.
Reaction rate constants for the formation of arynes
from aryl halides in diethyl ether at 20 ° C; 10 6 · k 2 [l · mol −1 · s −1 ]C 6 H 5 F C 6 H 5 Cl C 6 H 5 Br C 6 H 5 I. Phenyllithium 40.8 4.0 4.9 2.8 Lithium piperidide
(without piperidine )860 275 440 168
If amines are added to the reaction mixture, for example as lithium piperidide / piperidine, the reaction rates change as a function of the amine concentration. In the case of bromobenzene , the reaction rate increases, while conversely it decreases in the case of fluorobenzene. By reducing the activation energy of the metalation reaction (1st step) in the case of fluoroaromatic compounds, this becomes increasingly reversible, which slows down or prevents the formation of aryne accordingly. In the reaction of fluoronaphthalene with lithium piperidide / piperidine, the substitution reaction then increasingly proceeds via the addition-elimination mechanism instead of via the aryne intermediate, as the piperidine content increases. This can also explain the resistance of fluorobenzene to a substitution reaction with potassium amide in ammonia. Investigations on deuterated fluorobenzene have shown that the metalation step can proceed quite normally. The intermediate product is then, due to the high concentration of ammonia, converted back into (then deduterated) fluorobenzene. Rolf Huisgen was able to show that the reaction rate of aryne formation in bromobenzene derivatives also depends on other ring substituents. For example, the reaction rate for a (further) bromine substituent in the ortho position is 140 times and for a fluorine substituent is 34 times, while alkyl radicals reduce the reaction rate. If the other substituent is in the meta position to bromine, the reaction rate with bromine is 600 times, with fluorine even 1700 times as high. If there is another substituent in the para position to the bromine, the change in the reaction rate is smallest.
Reactions / applications
Nucleophilic aromatic reactions
Arynes can react either as electrophiles or as nucleophiles , depending on the reaction partner . As electron-poor, i.e. electrophilic intermediates, they easily react with nucleophiles. Rolf Huisgen and Jürgen Sauer were able to show that nucleophilic, aromatic reactions often take place via arynes as intermediate stages. The reaction path via arynes competes with the path via an addition-elimination reaction. Among other things, the reaction conditions and the ring substituents decide which of the two routes is preferred. For example, when p -halo-toluenes (halogen = chlorine, bromine and iodine) are reacted with 4 molar sodium hydroxide solution at 340 ° C. to form cresol, the process takes place almost completely via an elimination reaction with an aryne intermediate, while at 250 ° C. the Addition mechanism has a significant share. An important indicator of which reaction mechanism takes place in an aromatic substitution is a change in the substitution pattern. If the newly entered group is exclusively on the same ring atom, one can assume a classic nucleophilic aromatic reaction. In this case, entering and leaving groups in the transition state are bonded to the same ring atom at the same time. In the case of an elimination reaction via arynes , the ortho (neighboring) ring atoms are also included instead , which always leads to a partial rearrangement. John D. Roberts investigated reactions on substituted aryl halides with sodium or potassium amide in liquid ammonia to form substituted anilines .
Remainder (R) Halogen (X) Total yield [%] Proportion ortho [%] Share meta [%] OCH 3 Br 33 0 100 CF 3 Cl 28 0 100 CH 3 Br 64 49 51 CH 3 Cl 64 45 55
Potassium amide can also act as a catalyst and accelerate the formation of the aryne. This is how reactions such as the conversion of chlorobenzene with the less reactive potassium anilide to di- or triphenylamine are made possible in the first place. Albert T. Bottini and John D. Roberts investigated the alkaline hydrolysis of chlorobenzene [1- 14 C], and found that, under the conditions of the technical process (15 percent NaOH, 370 ° C), the reaction preferably via the elimination process with a Arin runs as an intermediate stage. Depending on the reaction conditions chosen, in addition to phenol, diphenyl ether and o- and p- hydroxybiphenyl are also formed in different amounts .
Ring closure reactions
Rolf Huisgen and H. König reported on intramolecular ring closure reactions via aryne intermediates. An alkylarylamine, for example, was reacted with phenyllithium (C 6 H 5 Li). In this case, N- methyl-2,3-dihydro-indole is formed via the corresponding aryne in 58 percent yield. In contrast to this, the equivalent starting compound in which the alkyl group and the chlorine atom are in the ortho position to one another and which should exclusively supply the N- methyl-2,3-dihydroindole, forms a significantly smaller amount of product.
If the amino group contains another aromatic radical, phenanthridines can be synthesized via the aryne intermediate .
Similarly extend cyclization of o - or m -halo-acylanilides or -thioacylaniliden with potassium amide (KNH 2 ) to form an aromatic oxazole or thiazole ring.
Diels-Alder reactions
A reaction with arynes as an intermediate is the Diels-Alder reaction with dienes. Within a few years Georg Wittig reported on a whole series of Diels-Alder reactions of arynes, among others with cyclopentadiene (a), furan (b), pyrrole derivatives (c), benzene (d), anthracene (e) and cyclohexadiene ( f). Anthracene is converted from dehydrobenzene with the central benzene ring to triptycene by the Diels-Alder reaction . The pentiptycene is the anthracene analogues in the reaction with 1,2,4,5-tetrabromobenzene and butyl lithium .
By palladium - catalysts can with high 1,2-didehydrobenzenes regioselectivity to Triphenylenes be trimerized. In the presence of alkenes or alkynes, the palladium-catalyzed synthesis of substituted phenanthrene derivatives is also successful . Arynes react accordingly with vinyl indoles to form carbazoles . 1,2,4,5-Tetrabromobenzene reacts with butyllithium and furan to form 1,4,5,8-diepoxy-1,4,5,8-tetrahydroanthracene. The syn - and anti stereoisomers can use due to the different solubilities of methanol be separated.
Arylation reactions
If an aryl halide, such as chlorobenzene, is reacted with phenyllithium in boiling diethyl ether, biphenyl is formed from it , the reaction rate and the yield being relatively low. As mentioned above under illustration , the aryne is formed much faster with lithium piperidide. If lithium piperidide is added as a catalyst to the mixture of aryl halide and phenyllithium , the reaction is significantly accelerated and the yield is improved.
By varying the aryl chloride (Aryl 1 Cl) and the lithium aryl compound (Aryl 2 Li), it is possible to synthesize a number of partly unusual bisaryls.
Aryl 1 -chloride Aryl 2 -Lithium Response time in h Yield aryl 1 - aryl 2 without piperidine with piperidine Chlorobenzene Phenyllithium 9 17th 61 Chlorobenzene o-tolyllithium 2 6th 23 1-chloronaphthalene Phenyllithium 2 4th 29 1-chloronaphthalene Phenyllithium 9 22nd 66 2-chloronaphthalene Phenyllithium 2 10 50 2-chlorobiphenyl Phenyllithium 6th 18th 65 9-chlorophenanthrene Phenyllithium 1 13 58 9-chlorophenanthrene o-tolyllithium 1 14th 60 9-chlorophenanthrene Mesityllithium 40 9 36
Very bulky aryldialkylamines, such as N- naphthyldiisopropylamine, which are not accessible in other ways, can be prepared in good yields by arylation reactions via arynes .
Insertion reactions
With 2- (trimethylsilyl) aryl triflates, very mild aryne precursors are available with which selective insertion reactions can be carried out. The following reaction equation shows an insertion into a CC bond of malonic acid ester .
Eiji Shirakawa showed the possibility of inserting arynes into the C [BOND] N bonds of urea derivatives, and Akkattu T. Biju and Frank Glorius reported in 2010 on highly selective insertion reactions of arynes into the C [BOND] H bond of aldehydes . 4-bromobenzaldehyde could be converted into 4-bromobenzophenone with a yield of 98 percent. If 1,2-didehydrobenzene is reacted with magnesium thiolates, the corresponding aryl thiolates are obtained. The magnesium residue on the ring can be replaced by appropriate electrophiles.
Dehydrobenzene Conversions
The reaction of 1,2- to 1,3-didehydrobenzene was postulated to explain the pyrolysis (at 900 ° C) of the phenyl-substituted aryne precursor 1 to acenaphthylene 7 .
The reaction takes place via several reactive intermediates: The aryne 2 is prepared from the phenyl-substituted phthalic anhydride 1 and rearranges itself to vinylidene 3 with ring contraction, i.e. reduction of the cyclohexa to the cyclopenta ring . The resulting carbene undergoes a CH insertion reaction to give pentalenes 4 and subsequent cleavage of a bond to give vinylidene 5 . After cis-trans isomerization to 6 , a last insertion reaction follows to form acenaphthylene. The evidence of phenyl migration in aryne 2 from 1,2-didehydrobenzene to 1,3-didehydrobenzene is based on isotope migration . If the ipso -carbon atom is replaced by a 13 C in the intermediate stage, it is found again in the shown mechanism in acenaphthylene at the ipso-aryne position. The presence of 13 C in the bridge position can only be explained if 15 percent isomerize from 2 to 1,3-didehydrobenzene A.
Tumor therapy
The formation of para-didehydrobenzene by Bergman cyclization from ene diynes is of particular research interest, as it can explain the mechanism of action of enediine cytostatics . The calicheamicins , esperamicins and dynemicins used in cancer treatment act via the reactive intermediate stages of para-didehydrobenzene derivatives and can be used specifically to break the sugar chains of the DNA double strands in vivo , which leads to cell death. Thanks to their special molecular structure, the Bergmann cyclization can take place at room temperature. Since the naturally occurring en-diines are highly toxic, derivatives must be developed which have maximum toxicity towards cancer cells with low toxicity towards healthy cells.
More options
The chemistry of the arynes was used to synthesize novel arylamines in a tandem reaction including two Diels-Alder reactions with three dehydrobenzene molecules that react with an imidazole :
Web links
literature
- Norman L. Allinger , Michael P. Cava , Don C. de Jongh , CR Johnson , NA Lebel , Calvin L. Stevens : Organic Chemistry. Walter de Gruyter, Berlin 1980, ISBN 3-11-004594-X , pp. 650-652.
- Hans Beyer , Wolfgang Walter : Textbook of organic chemistry. 19th edition. S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , pp. 447-448.
- RT Morrison, RN Boyd: Textbook of Organic Chemistry. 3. Edition. Verlag Chemie, Weinheim 1986, ISBN 3-527-26067-6 , pp. 1131-1136.
- A. Streitwieser. CH Heathcock: Organic Chemistry. Verlag Chemie, Weinheim 1980, ISBN 3-527-25810-8 , pp. 1058-1060.
- Kurt PC Vollhardt , Neil E. Schore: Organic chemistry. 4th edition. Wiley-VCH, Weinheim 2005, ISBN 3-527-31380-X , pp. 1170-1172.
- Francis A. Carey, Richard J. Sundberg: Advanced Organic Chemistry, Part A: Structure and Mechanism. 5th edition. Plenum, New York, 2007, ISBN 978-0-387-44897-8 , pp. 821-824.
- Reinhard W. Hoffmann : Dehydrobenzene and Cycloalkynes. Academic Press, 1968, ISBN 0-12-352050-9 .
- Paul Rademacher: Lecture Notes Organic Chemistry IV: Reactive Intermediates (OCIV) (PDF; 4.9 MB)
- Hans Henning Wenk: Matrix isolation of fluorinated didehydrobenzenes and diradicals. Dissertation. Bochum 2002, DNB 964588234 , urn : nbn: de: hbz: 294-4854 . (PDF; 4 MB)
- Hans Peter Latscha, Uli Kazmaier, Helmut Alfons Klein: Organic chemistry: Chemistry basic knowledge II. 5th edition. Springer Verlag, Berlin 2002, ISBN 3-540-42941-7 , pp. 119-120.
- Peter Sykes: How do organic reactions work? 2nd Edition. Wiley-VCH, Weinheim 2001, ISBN 3-527-30305-7 .
- Peter Sykes: Reaction Mechanisms in Organic Chemistry. 7th edition. Verlag Chemie, Weinheim 1979, ISBN 3-527-21047-4 .
Individual evidence
- ^ TC Gilchrist, CW Rees: Carbenes, Nitrenes and Arynes. Th. Nelson and Sons, London 1969, ISBN 0-306-50026-4 .
- ^ Stefan Leisering, Christoph A. Schalley: tutorial reactivity and synthesis . Springer-Verlag, Berlin 2017, ISBN 978-3-662-53852-4 , pp. 188 ( limited preview in Google Book search).
- ^ Hans Peter Latscha, Uli Kazmaier, Helmut Alfons Klein: Organic chemistry . 6th edition. Springer-Verlag, Berlin / Heidelberg 2013, ISBN 978-3-540-77107-4 , p. 120 , doi : 10.1007 / 978-3-540-77107-4 .
- ↑ a b Hans Henning Wenk, Michael Winkler, Wolfram Sander: 100 years of didehydroaromatics . In: Angewandte Chemie . tape 115 , no. 5 , 2003, p. 518-546 , doi : 10.1002 / anie.200390119 .
- ^ WE Bachmann, HT Clarke: The mechanism of the Wurtz-Fittig reaction . In: J. Am. Chem. Soc. tape 49 , 1927, pp. 2089–2098 , doi : 10.1021 / ja01407a038 .
- ↑ JD Roberts, HE Simmons, LA Carlsmith, CW Vaughan: Rearrangement in the reaction of chlorobenzene-1-C14 with potassium amide . In: J. Am. Chem. Soc. tape 75 , 1953, pp. 3290–3291 , doi : 10.1021 / ja01109a523 .
- ↑ Erwin F. Jenny, John D. Roberts: About the mechanism of the formation of diphenyl from fluorobenzene and phenyllithium . In: Helvetica Chimica Acta . tape 38 , no. 5 , 1955, pp. 1248–1254 , doi : 10.1002 / hlca.19550380520 .
- ↑ a b Georg Wittig, Liselotte Pohmer: About the intermediate occurrence of dehydrobenzene . In: Chemical Reports . tape 89 , no. 5 , 1956, pp. 1334-1351 , doi : 10.1002 / cber.19560890539 .
- ^ A b I. P. Fisher, FP Lossing: Ionization Potential of Benzyne . In: J. Am. Chem. Soc. tape 85 , 1963, pp. 1018-1019 , doi : 10.1021 / ja00890a054 .
- ↑ Hans-Friedrich Grützmacher, Joachim Lohmann: Detection of 9.10-Dehydro-phenanthrene by pyrolysis mass spectrometry . In: Justus Liebig's Annals of Chemistry . tape 726 , 1969, p. 47-66 , doi : 10.1002 / jlac.19697260109 .
- ^ OL Chapman, K. Mattes, CL McIntosh, J. Pacansky, GV Calder, G. Orr: Photochemical transformations. LII. Benzyne . In: J. Am. Chem. Soc. tape 95 , no. 18 , 1973, p. 6134-6135 , doi : 10.1021 / ja00799a060 .
- ^ Juliusz G. Radziszewski, B. Andes Hess Jr., Rudolf Zahradnik: Infrared spectrum of o-benzyne: experiment and theory . In: J. Am. Chem. Soc. tape 114 , no. 1 , 1992, p. 52-57 , doi : 10.1021 / ja00027a007 .
- ↑ Hans F. Ebel, Reinhard W. Hoffmann: Detection of dehydrobenzene in the gas phase . In: Liebig's annals of chemistry . tape 673 , no. 1 , 1964, pp. 1-12 , doi : 10.1002 / jlac.19646730102 .
- ↑ Ralf Warmuth: 1,2-Didehydrobenzol: a strained alkyne or a cumulene? - NMR spectroscopic characterization in a molecular container . In: Angewandte Chemie . tape 109 , no. 12 , 1997, p. 1406-1409 , doi : 10.1002 / anie.19971091234 .
- ^ Ralf Warmuth: Inner-Phase Stabilization of Reactive Intermediates . In: Eur. J. Org. Chem. Volume 2001 , no. 3 , 2001, p. 423-437 , doi : 10.1002 / 1099-0690 (200102) 2001: 3 <423 :: AID-EJOC423> 3.0.CO; 2-2 .
- ^ Frank H. Köhler: Dehydrobenzene: a (organometallic) stabilization problem . In: Chemistry in Our Time . tape 11 , no. 6 , 1977, pp. 190–196 , doi : 10.1002 / ciuz.19770110605 .
- ↑ a b c M. E. Blake, KL Bartlett, M. Jones Jr .: A m-Benzyne to o-Benzyne Conversion Through a 1,2-Shift of a Phenyl Group . In: J. Am. Chem. Soc. tape 125 , no. 50 , 2003, p. 6485-6490 , doi : 10.1021 / ja0213672 .
- ↑ AL Polishchuk, KL Bartlett, LA Friedman, M. Jones Jr .: A p-Benzyne to m-Benzyne Conversion Through a 1,2-Shift of a Phenyl Group. Completion of the Benzyne Cascade . In: J. Phys. Org. Chem. Band 17 , 2004, p. 798-806 , doi : 10.1002 / poc.797 .
- ^ Armin Schweig, Norbert Münzel, Hermann Meyer, Andreas Heidenreich: The electronic spectrum of o-benzyne . In: Structural Chemistry . tape 15 , no. 1 , p. 89-100 , doi : 10.1007 / BF00675788 .
- ↑ Anita M. Orendt, Julio C. Facelli, Juliusz G. Radziszewski, W. James Horton, David M. Grant, Josef Michl: 13 C dipolar NMR Spectrum of Matrix Isolated o-Benzyne-1,2- 13 C 2 . In: J. Am. Chem. Soc. tape 118 , no. 4 , 1996, pp. 846-852 , doi : 10.1021 / ja953417r .
- ^ Martin A. Bennett, Heinz P. Schwemlein: Metal complexes with small cycloalkynes and arynes . In: Angewandte Chemie . tape 101 , no. 10 , 1989, pp. 1349-1373 , doi : 10.1002 / anie.19891011006 .
- ^ FGA Stone: Advances in Organometallic Chemistry, Volume 42 . Academic Press, 1998, ISBN 0-12-031142-9 ( pages 148-186 in Google Book Search).
- ↑ Sabrina Brait, Stefano Deabate, Selby AR Knox, Enrico Sappa: The Coordination and Transformation of Arene Rings by Transition Metal Carbonyl Cluster Complexes . In: Journal of Cluster Science . tape 12 , no. 1 , 2001, p. 139-173 , doi : 10.1023 / A: 1016627113620 .
- ↑ Götz Bucher, Wolfram Sander, Elfi Kraka, Dieter Cremer: 2,4-Didehydrophenol - first IR spectroscopic detection of a meta-aryne . In: Angewandte Chemie . tape 104 , no. 9 , 1992, pp. 1225-1228 , doi : 10.1002 / anie.19921040916 .
- ↑ Ralph Marquardt, Wolfram Sander, Elfi Kraka: 1,3-Didehydrobenzol (meta-gasoline) . In: Angewandte Chemie . tape 108 , no. 7 , 1996, pp. 825-827 , doi : 10.1002 / anie.19961080719 .
- ↑ Wolfram Sander, Michael Exner, Michael Winkler, Andreas Balster, Angelica Hjerpe, Elfi Kraka, Dieter Cremer: Vibrational Spectrum of m-Benzyne: A Matrix Isolation and Computational Study . In: J. Am. Chem. Soc. tape 124 , no. 4 , 2002, p. 13072-13079 , doi : 10.1021 / ja012686g .
- ^ A b Richard R. Jones, Robert G. Bergman: p-Benzyne. Generation as an intermediate in a thermal isomerization reaction and trapping evidence for the 1,4-benzenediyl structure . In: J. Am. Chem. Soc. tape 94 , no. 2 , 1972, p. 660-661 , doi : 10.1021 / ja00757a071 .
- ↑ Thomas P. Lockhart, Paul B. Comita, Robert G. Bergman: Kinetic evidence for the formation of discrete 1,4-dehydrobenzene intermediates. Trapping by inter- and intramolecular hydrogen atom transfer and observation of high-temperature CIDNP . In: J. Am. Chem. Soc. tape 103 , no. 14 , 1981, pp. 4082-4090 , doi : 10.1021 / ja00404a018 .
- ↑ KC Nicolaou, W.-M. Dai: Chemistry and Biology of Endiin-Cytostatica / Antibiotica . In: Angewandte Chemie . tape 103 , no. 11 , 1991, p. 1453-1481 , doi : 10.1002 / anie.19911031106 .
- ↑ Michael Klein: Synthesis, structure and properties of functionalized cyclic enediynes and investigation of electronic substituent effects on the Bergman cyclization of acyclic enediynes. Dissertation. Regensburg 2003. (PDF; 1.4 MB)
- ↑ Ralph Marquardt, Andreas Balster, Wolfram Sander, Elfi Kraka, Dieter Cremer, J. George Radziszewski: 1,4-Didehydrobenzol . In: Angewandte Chemie . tape 110 , no. 7 , 1998, pp. 1001-1005 , doi : 10.1002 / (SICI) 1521-3757 (19980403) 110: 7 <1001 :: AID-ANGE1001> 3.0.CO; 2-D .
- ↑ Th. Kauffmann: The Hetarine . In: Angewandte Chemie . tape 77 , no. 13 , 1965, p. 557-571 , doi : 10.1002 / anie.19650771302 .
- ↑ Thomas Kauffmann, Rolf Wirthwein: Advances in the Hetarin area . In: Angewandte Chemie . tape 83 , no. 1 , 1971, p. 21-34 , doi : 10.1002 / anie.19710830104 .
- ↑ Thomas Kauffmann, Heinz Fischer, Reinhard Nürnberg, Rolf Writhwein: Hetarine, XIV About the selectivity of hetero- and carbocyclic arynes towards bases . In: Justus Liebig's Annals of Chemistry . tape 731 , no. 1 , 1970, p. 23-36 , doi : 10.1002 / jlac.19707310105 .
- ↑ Th. Kauffmann, R. Nürnberg, J. Schulz, R. Stabba: Hetarine, X detection of a 5-ring arine (1-methyl-4,5-dehydroimidazole) . In: Tetrahedron . tape 8 , no. 43 , 1967, p. 4273-4280 , doi : 10.1016 / S0040-4039 (00) 73013-9 .
- ↑ Christopher J. Cramer, Stefan Debbert: Heteroatomic substitution in aromatic σ biradicals: the six pyridynes . In: Chem. Phy. Lett. tape 287 , no. 3–4 , 1998, pp. 320-326 , doi : 10.1016 / S0009-2614 (98) 00192-4 .
- ↑ Th. Kauffmann, J. Hansen, K. Udluft, R. Wirthwein: Investigations on heterocyclic arynes . In: Angewandte Chemie . tape 76 , no. 13 , 1964, pp. 590 , doi : 10.1002 / anie.19640761375 .
- ↑ a b c Georg Wittig: 1.2-Dehydrobenzol . In: Angewandte Chemie . tape 77 , no. 17-18 , 1965, pp. 752-759 , doi : 10.1002 / anie.19650771704 .
- ↑ a b c Rolf Huisgen, H. König: Ring-closing reactions via Arine . In: Angewandte Chemie . tape 69 , no. 8 , 1957, pp. 268 , doi : 10.1002 / anie.19570690811 .
- ↑ a b c d Rolf Huisgen, Jürgen Sauer: Nucleophile aromatic substitutions via arynes . In: Angewandte Chemie . tape 72 , no. 3 , 1960, p. 91-108 , doi : 10.1002 / anie.19600720302 .
- ↑ Georg Wittig, Reinhard W. Hoffmann: Dehydrobenzol from 1.2.3-Benzothiadiazol-1.1-dioxide . In: Chemical Reports . tape 95 , no. 11 , 1962, pp. 2718-2728 , doi : 10.1002 / cber.19620951120 .
- ↑ Martin Stiles, Roy G. Miller: DECOMPOSITION OF BENZENEDIAZONIUM-2-CARBOXYLATE . In: J. Am. Chem. Soc. tape 82 , no. 14 , 1960, p. 3802-3802 , doi : 10.1021 / ja01499a094 .
- ↑ a b Jan Bülle, Aloys Hüttermann: The basic knowledge of organic chemistry: The most important organic reactions in the laboratory and in nature . Georg Thieme Verlag, 2000, ISBN 3-13-119041-8 ( page 81 in the Google book search).
- ^ Georg Wittig, Hans F. Ebel: on the occurrence of dehydrobenzene in photochemical and thermal processes . In: Liebig's annals of chemistry . tape 650 , no. 1 , 1961, pp. 20-34 , doi : 10.1002 / jlac.19616500103 .
- ↑ Ioannis Sapountzis, Wenwei Lin, Markus Fischer, Paul Knochel: Synthesis of functionalized arynes starting from 2-magnesiated diarylsulfonates . In: Angewandte Chemie . tape 116 , no. 33 , 2004, pp. 4464-4466 , doi : 10.1002 / anie.200460417 .
- ↑ a b Rolf Huisgen, Jürgen Sauer: Nucleophile aromatic substitutions, VIII. Kinetics of the release of benzin from halobenzenes . In: Chemical Reports . Volume 92, No. 1 , 1959, p. 192–202 , doi : 10.1002 / cber.19590920122 .
- ↑ R. Huisgen, W. Mack, L. Möbius: The proof of the intermediate stage in nucleophilic aromatic substitutions with elimination; to the structure of the arine . In: Tetrahedron . tape 9 , no. 1-2 , 1960, pp. 29-39 , doi : 10.1016 / 0040-4020 (60) 80049-X .
- ^ Georg Wittig, Liselotte Pohmer: About the intermediate occurrence of dehydrobenzene . In: Chemical Reports . tape 89 , no. 5 , 1956, pp. 1334-1351 , doi : 10.1002 / cber.19560890539 .
- ^ Rolf Huisgen, Jürgen Sauer: Reaction of the halogen naphthalenes with lithium piperidide . In: Angewandte Chemie . tape 69 , no. 11 , 1957, pp. 390 , doi : 10.1002 / anie.19570691109 .
- ↑ George E. Hall, Richard Piccolini, John D. Roberts: Exchange Reactions of Deuterated Benzene Derivatives with Potassium Amide in Liquid Ammonia . In: J. Am. Chem. Soc. tape 77 , no. 17 , 1955, pp. 4540–4543 , doi : 10.1021 / ja01622a033 .
- ↑ Rolf Huisgen, Wilhelm Mack, Klaus Herbig, Nele Ott, Elisabeth Anneser: Nucleophile aromatic Substitutionen, XIV. Partial rate constants for aryne formation from bromoaromatics using lithium piperidide . In: Chemical Reports . tape 93 , no. 2 , 1960, p. 412-424 , doi : 10.1002 / cber.19600930222 .
- ^ Arynes as electrophilic reagents, 1st edition: Reaction with alkylene-phosphoranes . In: Monthly magazine for chemistry . tape 75 , no. 6 , 1964, pp. 1759-1780 , doi : 10.1007 / BF00901736 .
- ↑ John D. Roberts, C. Wheaton Vaughan, LA Carlsmith, Dorothy A. Semenow: Orientation in Aminations of Substituted Halobenzenes . In: J. Am. Chem. Soc. tape 78 , no. 3 , 1956, pp. 611-614 , doi : 10.1021 / ja01584a025 .
- ^ FW Bergstrom, Richard E. Wright, Charles Chandler, WA Gilkey: THE ACTION OF BASES ON ORGANIC HALOGEN COMPOUNDS. I. THE REACTION OF ARYL HALIDES WITH POTASSIUM AMIDE . In: J. Org. Chem. Band 1 , no. 2 , 1936, pp. 170-178 , doi : 10.1021 / jo01231a007 .
- ^ Albert T. Bottini, John D. Roberts: Mechanisms for Liquid Phase Hydrolyses of Chlorobenzene and Halotoluenes . In: J. Am. Chem. Soc. tape 79 , no. 6 , 1957, pp. 1458–1462 , doi : 10.1021 / ja01563a050 .
- ↑ Kurt H. Meyer, Friedrich Bergius: About the representation of phenol from chlorobenzene . In: Reports of the German Chemical Society . tape 47 , no. 3 , 1914, pp. 3155-3160 , doi : 10.1002 / cber.191404703117 .
- ↑ Horst König, Rolf Huisgen: Nucleophile aromatic substitutions, XI. Further ring closure reactions via arynes . In: Chemical Reports . tape 92 , no. 2 , 1959, p. 429-441 , doi : 10.1002 / cber.19590920227 .
- ↑ Rolf Huisgen, Horst König, Arthur R. Lepley: Nucleophile aromatic Substitutionen, XVIII. New ring closings over Arine . In: Chemical Reports . tape 93 , no. 7 , 1960, pp. 1496-1506 , doi : 10.1002 / cber.19600930708 .
- ↑ SV Kessar: Some new aspects of benzyne and radical mediated cyclisations . In: Journal of Chemical Sciences . tape 100 , no. 2-3 , 1988, pp. 217-222 ( PDF ).
- ↑ Bjorn F. Hrutford, JF Bunnett: A GENERAL PRINCIPLE FOR THE SYNTHESIS OF HETEROCYCLIC AND HOMOCYCLIC COMPOUNDS . In: J. Am. Chem. Soc. tape 80 , no. 8 , 1958, pp. 2021-2022 , doi : 10.1021 / ja01541a065 .
- ↑ a b c d Georg Wittig: Formation and reactions of dehydrobenzene (cyclohexadienine) . In: Angewandte Chemie . tape 69 , no. 8 , 1957, pp. 245-251 , doi : 10.1002 / anie.19570690802 .
- ↑ Georg Wittig, Erhard Knauss: Dehydrobenzol and Cyclopentadiene . In: Chemical Reports . Volume 91, No. 5 , 1958, pp. 895-907 , doi : 10.1002 / cber.19580910502 .
- ↑ Georg Wittig, Wolfgang Behnisch: Dehydrobenzol and N-methyl-pyrrole . In: Chemical Reports . Volume 91, No. 11 , 1958, pp. 2358-2365 , doi : 10.1002 / cber.19580911116 .
- ^ Martin Stiles, Roy G. Miller, Reaction of Benzyne with Benzene and Naphthalene . In: J. Am. Chem. Soc. tape 85 , no. 12 , 1963, pp. 798–1800 , doi : 10.1021 / ja00895a023 .
- ^ Georg Wittig, Renate Ludwig: Triptycene from anthracene and dehydrobenzene . In: Angewandte Chemie . tape 68 , no. 1 , 1956, pp. 40 , doi : 10.1002 / anie.19560680107 .
- ↑ Harold Hart: Iptycenes, cuppedophanes and cappedophanes . In: Pure & App Chem . tape 65 , 1993, pp. 27-34 , doi : 10.1002 / jlac.19646730102 . Article (PDF; 989 kB)
- ↑ Diego Peña, Sonia Escudero, Dolores Pérez, Enrique Guitián, Luis Castedo: The First Efficient Palladium-Catalyzed Cyclotrimerization of Arynes: Synthesis of Triphenylenes . In: Angewandte Chemie . tape 110 , no. 19 , 1998, pp. 2804-2806 , doi : 10.1002 / (SICI) 1521-3757 (19981002) 110: 19 <2804 :: AID-ANGE2804> 3.0.CO; 2-2 .
- ↑ Enrique Guitián, Dolores Pérez, Diego Peña: Palladium-Catalyzed Cycloaddition Reactions of Arynes . In: Palladium in Organic Synthesis, Topics in Organometallic Chemistry . tape 14 , 2005, pp. 194-206, , doi : 10.1007 / b104128 .
- ↑ Eiji Yoshikawa, Yoshinori Yamamoto: Controlled palladium-catalyzed intermolecular dehydrobenzene-dehydrobenzene-alkene and dehydrobenzene-alkyne-alkene insertion - synthesis of phenanthrene and naphthalene derivatives . In: Angewandte Chemie . tape 112 , no. 1 , 2000, pp. 185-187 , doi : 10.1002 / (SICI) 1521-3757 (20000103) 112: 1 <185 :: AID-ANGE185> 3.0.CO; 2-N .
- ↑ Diego Peña, Dolores Pérez, Enrique Guitián: Insertion of arynes in σ-bonds . In: Angewandte Chemie . tape 118 , no. 22 , 2006, p. 3659-3661 , doi : 10.1002 / anie.200600291 .
- ↑ Ulf Pindur, Ludwig Pfeuffer, Manfred Eitel, Martina Rogge, Manfred Haber: Diels-Alder reactions of vinyl indoles with aryne and 1,4-benzoquinones: New potential DNA intercalators . In: Monthly books for chemistry / Chemical Monthly . tape 122 , no. 4 , 1991, pp. 291-298 , doi : 10.1007 / BF00810830 .
- ↑ Khalil Shahlai, Samuel Osafo Acquaah, Harold Hart: Use of 1,2,4,5-tetrabromobenzene as a 1,4-benzadiyne equivalent: anti- and syn-1,4,5,8-tetrahydroanthracene 1,4: 5 , 8-diepoxides In: Organic Syntheses . 75, 1978, p. 201, doi : 10.15227 / orgsyn.075.0201 ; Coll. Vol. 10, 2004, p. 678 ( PDF ).
- ↑ a b Rolf Huisgen, Jürgen Sauer, A. Hauser: Catalytic phenylation of chlorine aromatics with phenyllithium . In: Angewandte Chemie . tape 69 , no. 8 , 1957, pp. 267 , doi : 10.1002 / anie.19570690810 .
- ^ Rolf Huisgen, Jürgen Sauer, Alfred Hauser: Nucleophile aromatic Substitutionen, VI. Catalytic arylation of chlorinated aromatics . In: Chemical Reports . Volume 91, No. 11 , 1958, pp. 2366-2374 , doi : 10.1002 / cber.19580911117 .
- ↑ Rolf Huisgen, Ludwig Zirngibl: Nucleophile aromatic substitutions, VII. Steric and electronic factors in the addition of bases to 1,2-naphthin . In: Chemical Reports . Volume 91, No. 11 , 1958, pp. 2375-2382 , doi : 10.1002 / cber.19580911118 .
- ↑ Hiroto Yoshida, Masahiko Watanabe, Joji Ohshita, Atsutaka Kunai: Facile insertion reaction of arynes into carbon – carbon bonds . In: Chem. Commun. tape 26 , 2005, pp. 3292-3295 , doi : 10.1039 / B505392G .
- ↑ Hiroto Yoshida, Eiji Shirakawa, Yuki Honda, Tamejiro Hiyama: Addition of Ureas to Arynes: Straightforward Synthesis of Benzodiazepine and Benzodiazocine Derivatives . In: Angewandte Chemie . tape 114 , no. 17 , 2002, p. 3381-3383 , doi : 10.1002 / 1521-3757 (20020902) 114: 17 <3381 :: AID-ANGE3381> 3.0.CO; 2-C .
- ↑ Akkattu T. Biju, Frank Glorius: An intermolecular hydroacylation of arynes catalyzed by N-heterocyclic carbenes . In: Angewandte Chemie . tape 122 , no. 50 , 2010, p. 9955–9958 , doi : 10.1002 / anie.201005490 .
- ↑ Wenwei Lin, Ioannis Sapountzis, Paul Knochel: Synthesis of functionalized aryl magnesium reagents by the addition of magnesium aryl thiolates and amides to aryne . In: Angewandte Chemie . tape 117 , no. 27 , 2005, pp. 4330-4333 , doi : 10.1002 / anie.200500443 .
- ^ Adrian L. Smith, KC Nicolaou: The Enediyne Antibiotics . In: J. Med. Chem. Volume 39 , no. 11 , 1996, pp. 2103-2117 , doi : 10.1021 / jm9600398 .
- ↑ Marc J. Schottelius, Peter Chen: 9,10-Dehydroanthracene: p-Benzyne-Type Biradicals Abstract Hydrogen Unusually Slowly . In: J. Am. Chem. Soc. tape 118 , no. 20 , 1996, pp. 4896-4903 , doi : 10.1021 / ja960181y .
- ↑ a b Samuel J. Danishefsky, Matthew D. Shair: Observations in the Chemistry and Biology of Cyclic Enediyne Antibiotics: Total Syntheses of calicheamicin γ 1 and A dynemicin . In: J. Org. Chem. Band 61 , no. 1 , 1996, p. 16-44 , doi : 10.1021 / jo951560x .
- ^ Elfi Kraka, Dieter Cremer: Computer Design of Anticancer Drugs. A New Enediyne Warhead . In: J. Am. Chem. Soc. tape 122 , no. 34 , 2000, pp. 8245-8264 , doi : 10.1021 / ja001017k .
- ↑ Chunsong Xie, Yuhong Zhang: A New Tandem Reaction of Benzyne: One-Pot Synthesis of Aryl Amines Containing Anthracene . In: Organic Letters . tape 9 , 2007, p. 781-784 , doi : 10.1021 / ol063017g .