Triptycene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
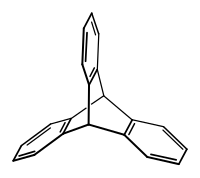
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triptycene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 20 H 14 | |||||||||||||||
| Brief description |
Colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 254.33 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
256 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Triptycene belongs to the class of aromatic hydrocarbons, the basic structure of which consists of the barrel . It is the formal product of a Diels-Alder reaction of anthracene with 1,2-didehydrobenzene, an aryne . The compound has a paddle wheel configuration with D 3h - symmetry . The hydrocarbon framework is extremely rigid, so that triptycene and triptycene derivatives, such as triptycenequinone, can be used in organic compounds as a molecular framework in the synthesis of certain molecular motors or as special ligand systems for, for example, hydrocyanation reactions .
history
Paul D. Bartlett and colleagues published the synthesis in 1942 and named it after "The triptych of antiquity", translated as "The Triptych of Antiquity", a book consisting of three pages attached to an axis.
Extraction and presentation
The original synthesis of triptycene took place in seven steps, starting with anthracene and p -benzoquinone . Triptycene can be produced from anthracene and anthranilic acid in the laboratory . The reactive and short-lived benzyne is produced using amyl nitrite and anthranilic acid, which reacts in situ with anthracene after a Diels-Alder reaction on the central benzene ring of the anthracene to form triptycene.
Individual evidence
- ↑ a b entry on triptycene. In: Römpp Online . Georg Thieme Verlag, accessed on September 30, 2014.
- ↑ a b Data sheet Triptycene, 98% from Sigma-Aldrich , accessed on February 27, 2013 ( PDF ).
- ↑ Triptycene quinones in synthesis: preparation of triptycene bis-cyclopentenedione Spyros Spyroudis and Nikoletta Xanthopoulou Arkivoc 2003 (vi) 95-105 Online article (PDF; 186 kB).
- ↑ Kelly TR, De Silva H, Silva RA: Unidirectional rotary motion in a molecular system . In: Nature . 401, No. 6749, September 1999, pp. 150-152. doi : 10.1038 / 43639 . PMID 10490021 .
- ↑ Bini L, Müller C, Wilting J, von Chrzanowski L, Spek AL, Vogt D: Highly selective hydrocyanation of butadiene toward 3-pentenenitrile . In: J. Am. Chem. Soc. . 129, No. 42, October 2007, pp. 12622-12623. doi : 10.1021 / ja074922e . PMID 17902667 . In this reaction, the substrate is 1,3-butadiene , the reactant is acetone cyanohydrin , the catalyst Ni (cod) 2 and the ligand is a bidentate organophosphine with a large grip angle on a triptycene framework.