Synthetic molecular engine
Synthetic molecular motors are molecular machines that are able to rotate when energized. Although the term molecular motor originally meant a naturally occurring protein that generates movement (via protein dynamics ), some research groups use the term to refer to non-biological synthetic motors. Many chemists follow the synthesis of such engines. The idea of the synthetic molecular motor was first mentioned by Richard Feynman in his 1959 speech There's Plenty of Room at the Bottom .
The basic requirements for a synthetic motor are repeated 360-degree movement, energy consumption and directional rotation. The first successes in the field were the chemical motor by T. Ross Kelly et al., Which only performed a non-repeatable rotation of 120 degrees, and the light-powered motor by Ben Feringa et al., Published in 1999 in the same issue of Nature published. Ben Feringa received the Nobel Prize in Chemistry in 2016 for this development . In 2008, Petr Král and co-workers proposed an electron tunnel motor that rotates continuously, thus opening up the possibility of practical implementation of a molecular machine. It is expected that the number of reports of successes in the field will increase as understanding of chemistry and physics at the nanoscale improves.
Naturally occurring molecular motors can be found, for example, in muscles ( motor proteins ) and in the flagellum of bacteria.
Chemically driven rotary motors
An example of a chemically powered rotary motor was reported by Kelly et al. Described in 1999. The system was constructed from a triptycene and a helicene and is able to carry out a directional rotation. The rotation takes place in five steps:
The amine group of the tritycene compound is substituted by an isocyanate group by condensation with phosgene ( a ). Thermal or spontaneous rotation around the central bond brings the isocyanate group close to the hydroxyl group located in the helicene half ( b ), allowing these two groups to react with each other ( c ). This irreversible reaction forms the system into a chain, a cyclic carbamate , which is more energetic and therefore closer to the rotational energy barrier than the original state. Further reaction of the triptycene half therefore requires less activation energy to overcome the energy barrier, with chain ( d ) being formed. Finally, the cleavage of the sets urethane group, the amine and the alcohol group of the molecule-free ( e ). The result of this reaction sequence is a directional 120 ° rotation of the triptycene relative to the helicene unit. Additional forward and backward rotation of the triptycene is prevented by the helicene half, whose function is that of the paddle of a ratchet. The directional movement of the system is the result of the asymmetrical arrangement of the helicene compound, as is the chain of the urethane substituent formed in c . The chain can only be loosened by rotating the triptycene molecule clockwise d , because both the counterclockwise rotation and the reverse reaction are energetically unfavorable. From the point of view, the preference for a direction of rotation is determined by the arrangement of the functional groups and the shape of the helicene unit and thus depends on the design of the molecule and not on external factors.
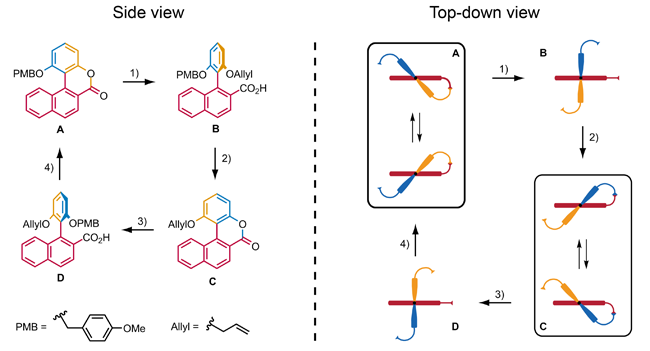
The Kelly et al. is a neat example of how chemical energy can be used to create directional rotational motion, a process similar to the consumption of adenosine triphosphate in living organisms to perform numerous processes. However, there is one disadvantage: the sequence of reactions that results in a 120 ° rotation is not repeatable. Kelly et al. have therefore looked for ways to expand the system so that this sequence of reactions can be repeated. But they were unsuccessful and gave up on the project. Two further examples of synthetic, chemically operated molecular rotary motors have been described in the literature that use a stereoselective ring-opening reaction of a racemic biaryl lactone with the aid of chiral reagents to produce a directional 90 ° rotation of one aryl relative to the other aryl. Branchud et al. published this experiment, which, followed by a ring closure reaction, can perform a simple 180 ° rotation. Feringa et al. used this experiment to develop a molecular structure that executes a 360 ° rotation. The whole rotation of this molecular motor takes place in four steps. In states A and C, rotation of the aryl is sterically hindered, although helical inversion is possible. In states B and D, the aryl can rotate relative to the naphthalene , with steric effects preventing the aryl from rotating past the naphthalene. Steps 1 and 3 are asymmetric ring-opening reactions that use a chiral reagent to control the direction of rotation of the aryl. Steps 2 and 4 consist of the deprotection of the phenol , followed by a regioselective ring closure reaction. So far, this molecular motor is the only example of a chemically operated rotary motor that can rotate 360 °.
Light-powered rotary molecular motors
In 1999 the laboratory of BLFeringa, University of Groningen, the Netherlands, reported on the development of a molecular motor. The 360 ° rotatable molecular motor consists of a bis- helicene , connected by an alkene double bond, which shows axial chirality and has two stereocenters . The cycle of directed rotation consists of four steps. The first step is a low-temperature endothermic photoisomerization of the trans- P, P -isomer 1 to the cis- M, M -isomer 2 , where P stands for the right-handed helix and M for the left-handed helix . In this course of the reaction, the two axial methyl groups are converted into two less sterically hindered, preferably equatorially bonded methyl groups. When cooled to 20 ° C, these methyl groups of the structure ( P, P ) shift back into the axial cis conformation 3 in an exothermic reaction, the helix inversion . Since the axial isomer is more stable than the equatorial isomer, the reverse reaction is blocked. A second photoisomerization transforms the P, P -cis structure 3 into the trans structure 4 , again with the formation of the sterically less favorable equatorial position. A thermal isomerization at 60 ° C closes the 360 ° cycle, shifting the methyl groups back into the axial position.
A bigger hurdle is the long reaction time with which the systems perform a complete rotation, which is not comparable with the speed with which motor proteins rotate in biological systems. In the fastest system, with fluorene in the lower half, the half-life for thermal helix inversion is 0.005 seconds. This compound is synthesized in a Barton-Kellog reaction. With this molecule, the slowest step of rotation is the thermal helix inversion, which was actually thought to be faster, since the isomer with the tert-butyl group is less stable than with the methyl group used. The unstable isomer is in fact more destabilized than the transition state that leads to helix inversion. The different behavior of the two isomers is illustrated by the fact that the half-life for the compound with the methyl group instead of the tert-butyl group is 3.2 minutes.
Feringa's principle was used for the prototype of a nano car . The synthesized car has a helicene-powered machine with an oligo-phenylethynylene body and four carborane wheels, and it is expected to be able to move on a solid surface, which is supposed to be observable in a scanning tunneling microscope , but so far it is still possible didn't happen. The engine will not work with fullerene wheels because they would erase the engine's photochemistry . Feringa motors have been shown to remain functional when chemically bonded to solid surfaces. The possibility of using certain Feringa systems as asymmetric catalysts could be demonstrated.
Experimental realization of an electric single-molecule motor
A single molecule electric motor has been described which consists of a single butyl dimethyl sulfoxide molecule. This molecule is absorbed by a copper single crystal (111) through chemisorption .
Nanoscopic observation of molecular rotation
A rotor and a stator are two components required to induce rotary motion. A rotor-stator pair can be produced by attaching propeller-shaped supramolecules to a nanochannel that serves as a fitting with a uniform diameter. An alternative to circular stators are hexagonal blades, which consist of molecules known from the field of two-dimensional molecular self-assembly and molecular nanotechnology . If you use hexagonal nanochannels on a silver substrate, you can observe molecular rotation in real time, analyze it on metal surfaces with scanning tunneling microscopy , and visualize it with molecular simulations.
literature
- T. Ross Kelly: Molecular Machines (= Topics in Current Chemistry . Volume 262 ). Springer Science & Business Media, 2005, ISBN 3-540-28501-6 , doi : 10.1007 / b105501 .
- M. Wilson, J. Solà, A. Carlone, S. Goldup, N. Lebrasseur & David A. Leigh: An autonomous chemically fueled small-molecule motor , Nature, 534, 235–240, (2016), doi: 10.1038 / nature18013
- S Kassem, T van Leeuwen, AS Lubbe, MR Wilson, BL Feringa and DA Leigh: Artificial molecular motors , Chem. Soc. Rev., 46, 2592-2621 (2017)
Individual evidence
- ↑ Jordan R. Quinn: Synthetic Molecular Motors . ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. scs.uiuc.edu, 2002, accessed November 18, 2015.
- ^ TR Kelly, H De Silva, RA Silva: Unidirectional rotary motion in a molecular system . In: Nature . 401, No. 6749, 1999, pp. 150-2. doi : 10.1038 / 43639 .
- ↑ T. Ross Kelly, Xiaolu Cai, Fehmi Damkaci, Sreeletha B. Panicker, Bin Tu, Simon M. Bushell, Ivan Cornella, Matthew J. Piggott, Richard Salives, Marta Cavero, Yajun Zhao, Serge Jasmin: Progress toward a Rationally Designed , Chemically Powered Rotary Molecular Motor . In: Journal of the American Chemical Society . 129, No. 2, 2007, p. 376. doi : 10.1021 / ja066044a . PMID 17212418 .
- ↑ Ying Lin, Bart J. Dahl, Bruce P. Branchaud: Net directed 180 ° aryl – aryl bond rotation in a prototypical achiral biaryl lactone synthetic molecular motor . In: Tetrahedron Letters . 46, No. 48, 2005, p. 8359. doi : 10.1016 / j.tetlet.2005.09.151 .
- ↑ SP Fletcher, F Dumur, MM Pollard, BL Feringa: A Reversible, Unidirectional Molecular Rotary Motor Driven by Chemical Energy . In: Science . 310, No. 5745, 2005, pp. 80-2. doi : 10.1126 / science.1117090 . PMID 16210531 .
- ↑ Ben L. Feringa, Nagatoshi Koumura, Robert WJ Zijlstra, Richard A. Van Delden, Nobuyuki Harada: Light-driven monodirectional molecular rotor . In: Nature . 401, No. 6749, 1999, p. 152. doi : 10.1038 / 43646 . PMID 10490022 .
- ↑ Javier Vicario, Martin Walko, Auke Meetsma, Ben L. Feringa: Fine Tuning of the Rotary Motion by Structural Modification in Light-Driven Unidirectional Molecular Motors . In: Journal of the American Chemical Society . 128, No. 15, 2006, p. 5127. doi : 10.1021 / ja058303m . PMID 16608348 .
- ↑ Javier Vicario, Auke Meetsma, Ben L. Feringa: Controlling the speed of rotation in molecular motors. Dramatic acceleration of the rotary motion by structural modification . In: Chemical Communications . No. 47, 2005, p. 5910. doi : 10.1039 / b507264f .
- ^ Jean-François Morin, Yasuhiro Shirai, James M. Tour: En Route to a Motorized Nanocar . In: Organic Letters . 8, No. 8, 2006, p. 1713. doi : 10.1021 / ol060445d . PMID 16597148 .
- ↑ Gregory T. Carroll, Michael M. Pollard, Richard Van Delden, Ben L. Feringa: Controlled rotary motion of light-driven molecular motors assembled on a gold film . In: Chemical Science . 1, 2010, p. 97. doi : 10.1039 / C0SC00162G .
- ↑ Gregory T. Carroll, Gábor London, Tatiana Fernández Landaluce, Petra Rudolf, Ben L. Feringa: Adhesion of photon-driven molecular motors to surfaces via 1, 3-dipolar cycloadditions: Effect of interfacial interactions on molecular motion . In: ACS Nano . 5, No. 1, 2011, pp. 622-30. doi : 10.1021 / nn102876j . PMID 21207983 .
- ^ J. Wang, BL Feringa: Dynamic Control of Chiral Space in a Catalytic Asymmetric Reaction Using a Molecular Motor Science . In: Science . 331, No. 6023, 2011, p. 1429. doi : 10.1126 / science.1199844 . PMID 21310964 .
- ^ T. Ooi: Heat and Light Switch a Chiral Catalyst and Its Products . In: Science . 331, No. 6023, 2011, pp. 1395-1396. doi : 10.1126 / science.1203272 . PMID 21415343 .
- ↑ Heather L. Tierney et al. a .: Experimental demonstration of a single-molecule electric motor . In: Nature Nanotechnology . tape 6 , no. 10 , October 2011, p. 625-629 , doi : 10.1038 / nnano.2011.142 .
- ↑ D. Kuhne, F. Klappenberger, W. Krenner, S. Klyatskaya, M. Ruben, JV Barth: Rotational and constitutional dynamics of caged supramolecules . In: Proceedings of the National Academy of Sciences . 107, No. 50, 2010, p. 21332. doi : 10.1073 / pnas.1008991107 .