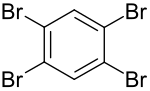
1,2,4,5-tetrabromobenzene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,2,4,5-tetrabromobenzene | |||||||||||||||
| other names |
sym. -Tetrabromobenzene |
|||||||||||||||
| Molecular formula | C 6 H 2 Br 4 | |||||||||||||||
| Brief description |
white to yellow-brown crystalline powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 393.70 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.518 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
180-182 ° C |
|||||||||||||||
| solubility |
practically insoluble in water, very easily soluble in diethyl ether , soluble in dimethyl sulfoxide , chloroform and in benzene |
|||||||||||||||
| Refractive index |
1.6303 (25 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,2,4,5-Tetrabromobenzene is a four-fold symmetrically bromine-substituted benzene . With its isomers 1,2,3,4-tetrabromobenzene and 1,2,3,5-tetrabromobenzene, it belongs to the group of tetrabromobenzenes .
It is a starting material for liquid crystals and OLED materials as well as mono- and bis arynes . 1,2,4,5-Tetrabromobenzene is an important degradation product of the completely brominated benzene hexabromobenzene, which is used as a flame retardant , in the animal organism and has liver-damaging properties.
Occurrence and representation
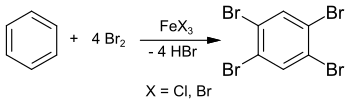
The synthesis of 1,2,4,5-tetrabromobenzene from benzene and excess bromine in a sealed tube at 150 ° C was reported as early as 1865. The significantly lower freezing point of approx. 160 ° C, however, indicates impurities in the end product.
In 1885, Adolf Scheufelen published in his dissertation the synthesis of 1,2,4,5-tetrabromobenzene in the presence of iron (III) chloride FeCl 3 as a catalyst and obtained the purer product (melting point 175 ° C.) “in beautiful needles ".
The synthesis can also take place in solution with chloroform or carbon tetrachloride and gives 1,2,4,5-tetrabromobenzene in 89% yield.
As a teaching example for electrophilic aromatic substitutions , this reaction can also be carried out in a laboratory experiment with excess bromine and iron nails (as the starting material for iron (III) bromide FeBr 3 ). 1,4-Dibromobenzene is formed as an intermediate stage , which reacts further with excess bromine to form 1,2,4,5-tetrabromobenzene.
use
Building block for liquid crystals and fluorescent dyes
The symmetrical substitution pattern with reactive bromine atoms makes 1,2,4,5-tetrabromobenzene an interesting starting compound for nematic liquid crystals with crossed mesogens
and for columnar (discotic) liquid crystals with an extended planar, “board-like” tetrabenzoanthracene ring system.
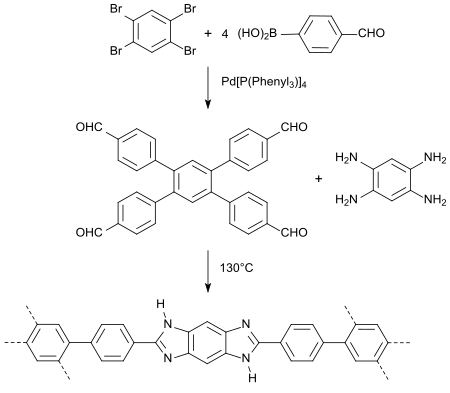
In a one-pot reaction may be selected from 1,2,4,5-tetrabromobenzene with the aromatic aldehyde 4-hydroxybenzaldehyde , the alkylating agent 1-bromopentane , the Wittig reagent methyltriphenylphosphonium iodide, the base potassium carbonate , the phase transfer catalyst is tetrabutylammonium bromide , the rear reagent palladium (II) acetate and the Heck cocatalyst 1,3-bis (diphenylphosphino) propane (dppp) in dimethylacetamide, a symmetrical tetraalkoxylstilbene can be obtained directly as E isomer in 17% yield.
Such compounds are of interest because of their pronounced π conjugation as optical brighteners , OLED materials or liquid crystals.
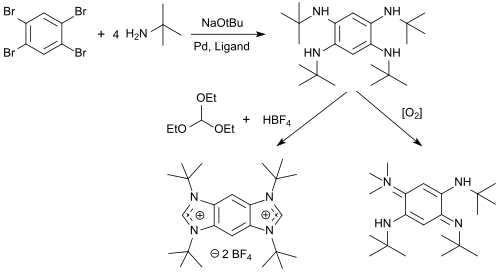
From 1,2,4,5-tetrabromobenzene, N-alkyl-tetraminobenzenes can be obtained in high yields, and these can be cyclized with triethyl orthoformate and acids to give benzobis (imidazolium) salts (BBI salts) and oxidized with oxygen to give 1,4-benzoquinonediimines .
The BBI salts are versatile fluorescent dyes with emission wavelengths λ em between 329 and 561 nm, pronounced solvatochromism and a strong solvent-dependent Stokes shift , which can be used as protein tags for fluorescent labeling of proteins.
Starting material for aryne
A 1,4-monoarine can be prepared in situ from 1,2,4,5-tetrabromobenzene with one equivalent of n- butyllithium by bromine abstraction, which with furan immediately forms 6,7-dibromo-1,4-epoxy-1 , 4-dihydronaphthalene (6,7-dibromonaphthalene-1,4-endoxide) is trapped in 70% yield.
When using 2,5-dialkylfurans, such as. B. 2,5- (Di-n-octyl) furan, the dibrominated monoendoxide is formed in 64% yield, from which the 2,3-dibromo-5,8-di-n-octylnaphthalene with titanium tetrachloride / zinc dust 88% yield results.
With titanium tetrachloride / zinc dust, the end oxide can be reduced to 2,3-dibromonaphthalene in 86% yield.
The end oxide reacts with 3-sulfolene in a Diels-Alder reaction with elimination of sulfur dioxide to form a tricyclic adduct from which 2,3-dibromoanthracene can be obtained in good yield.
If the dibromo endoxide is allowed to react with further furan, the tricyclic 1,4-bis-adduct 1.4: 5.8 is formed in 71% yield in the presence of n-butyllithium or potassium amide via an intermediate 1,4-aryne -Diepoxy-1,4,5,8-tetrahydroanthracene as a syn - anti mixture .
With sodium amide in ethylene glycol dimethyl ether DME, on the other hand, the dibromoendoxide behaves as a 1,3-aryne equivalent and forms a phenanthrene- like tricyclic 1,3-bis-adduct with furan, which with the action of sodium amide with furan to form a triphenylene derivative (1 , 3,5-tris-arene) can be trapped.
[2 + 4] cycloadditions with 1,2,4,5-tetrabromobenzene sometimes run in very high yields, such as B. a dihalosubstituted 1,3-diphenyl-isobenzofuran to a tetrahalogenated anthracene derivative (98%), which successively with 1,3-diphenyl-isobenzofuran with 65% yield to a pentacene and this with furan to a hexacene derivative (67%) can be transferred.
The crosslinking of benzimidazole-modified polymers provides materials with a high absorption capacity for carbon dioxide , which could be suitable for separating CO 2 from gas mixtures.
safety instructions
1,2,4,5-Tetrabromobenzene is a liver-toxic breakdown product of the flame retardant hexabromobenzene and was detected in breast milk samples in Japan as early as 1987.
Individual evidence
- ↑ Entry on 1,2,4,5-Tetrabromobenzene at TCI Europe, accessed on July 7, 2017.
- ↑ a b c d data sheet 1,2,4,5-tetrabromobenzene from Sigma-Aldrich , accessed on July 7, 2017 ( PDF ).
- ^ A b Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 88 .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2017, ISBN 978-1-4987-5429-3 , pp. 3-494 .
- ↑ Entry on 1,2,4,5-Tetrabromobenzene at Toronto Research Chemicals , accessed on July 7, 2017 ( PDF ).
- ↑ a b A. Scheufelen: About iron compounds as bromine carriers . In: Liebigs Ann. Chem. Band 231 , no. 2 , 1885, p. 152-195 , doi : 10.1002 / jlac.18852310204 .
- ↑ a b c H. Hart, A. Bashir-Hashemi, J. Luo, MA Meador: Iptycenes: Extended triptycenes . In: Tetrahedron . tape 42 , no. 6 , 1986, pp. 1641-1654 , doi : 10.1016 / S0040-4020 (01) 87581-5 .
- ↑ K. Shahlai, SO Acquaah, H. Hart: USE OF 1,2,4,5-TETRABROMOBENZENE AS A 1,4-BENZADIYNE EQUIVALENT: anti- AND syn-1,4,5,8-TETRAHYDROANTHRACENE 1,4: 5,8-DIEPOXIDES In: Organic Syntheses . 75, 1998, p. 201, doi : 10.15227 / orgsyn.075.0201 ; Coll. Vol. 10, 2004, p. 678 ( PDF ).
- ↑ E. Bruchajzer, B. Frydrych, JA Szymanska: Effect of repeated administration of hexabromobenzene and 1,2,4,5-tetrabromobenzene on the levels of selected cytochromes in rat liver . In: Int. J. Occup. Med. Environ. Health . tape 17 , no. 3 , 2004, p. 347-353 , doi : 10.1016 / S0040-4020 (01) 87581-5 .
- ↑ A. Riche, P. Bérard: About the bromine-containing derivatives of benzene and its homologues . In: Liebigs Ann. Chem. Band 133 , no. 1 , 1865, p. 51-54 , doi : 10.1002 / jlac.18651330106 .
- ↑ Patent US2979537 : Selective bromination of benzene. Applied on February 4, 1959 , published April 11, 1961 , applicant: The Dow Chemical Co., inventor: AA Asadorian.
- ^ B. Cox, DG Kubler, CA Wilson: Experiments with electrophilic aromatic substitution reactions . In: J. Chem. Educ. tape 54 , no. 6 , 1977, pp. 379 , doi : 10.1021 / ed054p379 .
- ↑ H.-H. Chen et al .: Enantiotropic nematics from cross-like 1,2,4,5-tetrakis (4'-alkyl-4-ethynylbiphenyl) benzenes and their biaxiality studies . In: Chemistry - A European Journal . tape 18 , no. 31 , 2012, p. 9543-9551 , doi : 10.1002 / chem . 201103453 .
- ^ S. Kumar: Chemistry of discotic liquid crystals: from monomers to polymers . CRC Press, Boca Raton, FL, USA 2011, ISBN 978-1-4398-1145-0 , pp. 200 .
- ↑ MC Artal, KJ Toyne, JW Goodby, J. Barbera, DJ Photinos: Synthesis and mesogenic properties of novel board-like liquid crystals . In: J. Mater. Chem. Band 11 , 2011, p. 2801-2807 , doi : 10.1039 / B105351P .
- ↑ KN Patel, BV Kamath, AV Bedekar: Synthesis of alkyloxy stilbenes by one-pot O-alkylation-Wittig and O-alkylation-Wittig-Heck reaction sequence . In: Tetrahedron Lett. tape 54 , no. 1 , 2013, p. 80-84 , doi : 10.1016 / tetlet.2012.10.102 .
- ↑ DM Khramov, AJ Boydston, CW Bielawski: Highly efficient synthesis and solid-state characterization of 1,2,4,5-tetrakis (alkyl- and arylamino) benzenes and cyclization to their respective benzobis (imidazolium) salts . In: Org. Lett. tape 8 , no. 9 , 2006, p. 1831-1834 , doi : 10.1021 / ol060349c .
- ↑ AJ Boydston: Modular fluorescent benzobis (imidazolium) saltes: Syntheses, photophysical analyzes, and applications . In: J. Am. Chem. Soc. tape 130 , no. 10 , 2008, p. 3143-3156 , doi : 10.1021 / ja7102247 .
- ^ Z. Chen, P. Müller, TM Swager: Syntheses of soluble, π-stacking tetracene derivatives . In: Org. Lett. tape 8 , no. 2 , 2006, p. 273-276 , doi : 10.1021 / ol0526468 .
- ↑ H. Hart, C.-Y. Lai, GC Nwokogu, S. Shamouilian: Tetrahalobenzenes as diaryne equivalents in polycyclic arene synthesis . In: Tetrahedron . tape 43 , no. 22 , 1987, pp. 5203-5224 , doi : 10.1016 / S0040-4020 (01) 87696-1 .
- ↑ C.-T. Lin, T.-C. Chou: Synthesis of 2,3-dibromoanthracene . In: Synthesis . tape 1988 , no. 8 , 1988, pp. 628-630 , doi : 10.1055 / s-1988-27659 .
- ↑ a b F. Raymo, FH Kohnke, F. Cardullo: The regioselective generation of arynes from polyhalogenobenzenes. An improved synthesis of syn - and anti -1,4,5,8,9,12-hexahydro-1,4: 5,8: 9,12-triepoxytriphenylene . In: Tetrahedron . tape 48 , no. 33 , 1992, pp. 6827-6838 , doi : 10.1016 / S0040-4020 (01) 89874-4 .
- ↑ H. Hart, N. Raju, MA Meador, DL Ward: Synthesis of heptiptycenes with face-to-face arene rings via a 2,3: 6,7-anthradiyne equivalent . In: J. Org. Chem. Band 48 , no. 23 , 1983, pp. 4357-4360 , doi : 10.1021 / jo00171a039 .
- ↑ S. Eda, T. Hamura: Selective Halogen-Lithium Exchange of 1,2-Dihaloarenes for Successive [2 + 4] Cycloadditions of Arynes and Isobenzofurans . In: Molecules . tape 20 , 2015, p. 19449-19462 , doi : 10.3390 / molecules201019449 .
- ↑ S. Altarawneh, S. Behera, P. Jena, HM El-Kaderi: New insights into carbon dioxide interactions with benzimidazole-linked polymers . In: Chem. Commun. tape 50 , 2014, p. 3571-3574 , doi : 10.1039 / C3CC45901B .
- ↑ T. Miyazaki, T. Yamagishi, M. Matsumoto: Determination and residual levels of 1,2,4,5-tetrabromobenzene and Mirex in human milk samples . In: Food Hygiene and Safety Science . tape 28 , no. 2 , 1987, pp. 125-129 , doi : 10.3358 / shokueishi.28.125 .