3-sulfolene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-sulfolene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 6 O 2 S | |||||||||||||||
| Brief description |
white to light yellow crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 118.15 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
hardly soluble in water (130 g / l (at 20 ° C), soluble in ethanol , benzene , diethyl ether and chloroform |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
3-Sulfolene is a monounsaturated cyclic sulfone , which is formed from 1,3-butadiene and sulfur dioxide (SO 2 ) in a (4 + 1) cheletropic reaction and at temperatures above 80 ° C in a [4 + 1] cyclo-elimination breaks down into the gaseous starting materials. It is therefore used as an easily manageable “solid” cis -1,3-butadiene and SO 2 and is widely used as a diene in Diels-Alder reactions .
Occurrence and representation
To produce 3-sulfolene, liquid 1,3-butadiene is mixed with an excess of liquid sulfur dioxide in an autoclave at approx. −20 ° C. in the presence of small amounts of a phenolic polymerization inhibitor, such as B. hydroquinone or pyrogallol , condensed and left to stand at room temperature for eight days or heated to about 130 ° C for 30 minutes.
After recrystallization from ethanol, the pure product is obtained in almost quantitative yield.
properties
3-Sulfolene is a white, odorless, crystalline, indefinitely stable solid that dissolves in water and many organic solvents. The occasionally reported pungent odor is likely due to adhering sulfur dioxide. The compound dissolves unchanged in concentrated acids, such as B. nitric acid or sulfuric acid and can even consist of conc. HNO 3 are recrystallized.
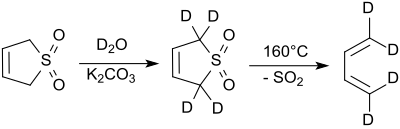
The hydrogen atoms in the 2- and 5-positions of 3-sulfolene can be exchanged quickly and completely by deuterium atoms (from deuterium oxide ) in alkaline media or under catalysis with sodium cyanide .
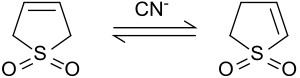
3-Sulfolene isomerizes under alkaline conditions or under catalysis with cyanide ions to form a mixture of 2-Sulfolene and 3-Sulfolene, the composition of which depends on the cyanide / sulfolene ratio.
At 50 ° C., an equilibrium between 42% 3-sulfolene and 58% 2-sulfolene is established. The thermodynamically more stable 2-sulfolene can be isolated from the isomer mixture by heating it for several days at 100 ° C because of the thermal decomposition of 3-sulfolene at temperatures above 80 ° C as a pure substance in the form of white plates (melting point 48-49 ° C).
At higher temperatures, especially above about 110 ° C, 3-sulfolene breaks down in a retro-cheletropic reaction to form pure cis -1,3-butadiene and sulfur dioxide.
With the in situ generation and immediate conversion of cis -1,3-butadiene, contact with the diene (flammable, carcinogenic, forming explosive mixtures with air) is largely avoided.
Applications
Reactions on 3-sulfolene
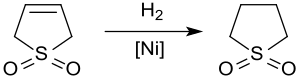
The hydrogenation of 3-sulfolene on Raney nickel at approx. 20 bar and 60 ° C. only gives sulfolane in yields of up to 65% because the catalyst is poisoned by sulfur compounds .
In aqueous solution, 3-sulfolene easily adds bromine to 3,4-dibromo-tetrahydrothiophene-1,1-dioxide, which could not be dehydrobrominated to thiophene-1,1-dioxide by H. Staudinger with silver carbonate . Via the detour of the formation of 3,4-bis (dimethylamino) tetrahydrothiophene-1,1-dioxide and successive double quaternization with methyl iodide and Hofmann elimination with silver hydroxide , the reactive thiophene-1,1-dioxide, which is only stable in solution, is accessible.
The two-step synthesis with double dehydrobromination of 3,4-dibromo-tetrahydrothiophene-1,1-dioxide with powdered sodium hydroxide in tetrahydrofuran (THF) or with metallic potassium dispersed by ultrasound is less laborious .
The solution of thiophene-1,1-dioxide in THF can be used directly in a [4 + 6] cycloaddition with 6-dimethylaminofulvene for the synthesis of the blue aromatic hydrocarbon azulene .
The pure yield of azulene is 33%.
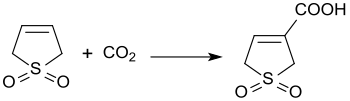
The reaction of 3-sulfolene with carbon dioxide at 3 bar pressure and in the presence of diazabicycloundecene produces 3-sulfolene-3-carboxylic acid in 45% yield.
With diazomethane , 3-sulfolene forms a fused five-membered ring system in a 1,3-dipolar cycloaddition with ring closure in 90% yield .
Diels-Alder reactions with 3-sulfolene
As early as 1938, Kurt Alder and co-workers reported on Diels-Alder adducts from the isomeric 2-sulfolene with 1,3-butadiene and 2-sulfolene with cyclopentadiene .
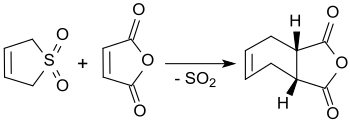
3-Sulfolene reacts with maleic anhydride in boiling xylene in yields of up to 90% to form cis-4-cyclohexene-1,2-dicarboxylic anhydride.
Even with dienophiles in the trans configuration, such as. B. diethyl reacts 3-sulfolene at 110 ° C with SO 2 elimination in 66- to 73% yield for the trans-4-cyclohexene-1,2-dicarboxylic acid diethyl ester.
6,7-dibromo-1,4-epoxy-1,4-dihydronaphthalene, which is available in 70% yield after debromination from 1,2,4,5-tetrabromobenzene by means of one equivalent of n-butyllithium and a Diels-Alder reaction with furan (6,7-Dibromonaphthalene-1,4-endoxide) reacts with 3-sulfolene in boiling xylene, a tricyclic adduct which, on treatment with perchloric acid, gives a dibromodihydroanthracene, which in the last stage with 2,3-dichloro-5,6- dicyano-1,4-benzoquinone (DDQ) is dehydrated to 2,3-dibromoanthracene.
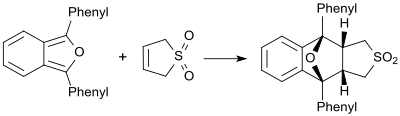
Compared to very reactive dienes, such as. B. 1,3-diphenylisobenzofuran, butadiene sulfone behaves as a dienophile and forms the corresponding Diels-Alder adduct.
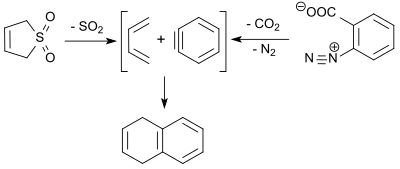
The 1,3-butadiene formed in the retro-cheletropic reaction of 3-sulfolene reacts with the dienophile dehydrobenzene ( benzyne ) obtained by thermal decomposition of benzenediazonium-2-carboxylate in 2-pentanone at 100 ° C. in 9% yield to 1,4-dihydronaphthalene.
Polymerization of 3-sulfolene
In their work from 1935, H. Staudinger and co-workers found that the reaction of butadiene and SO 2 at room temperature, in addition to crystalline butadiene sulfone (89% yield), forms an amorphous solid polymer in small quantities. By radical polymerization of butadiene sulfone in peroxide-containing diethyl ether, they obtained up to 50% insoluble high molecular weight polybutadiene sulfones, which were also stable in concentrated nitric and sulfuric acid.
In later investigations 3-sulfolene could be polymerized neither ionically nor radically at temperatures below 80 ° C, but above 100 ° C and with the radical initiator azobis (isobutyronitrile) (AIBN) to polybutadiene sulfone. On the other hand, 3-sulfolene does not copolymerize with vinyl compounds . In contrast, 2-sulfolene does not homopolymerize , but forms with vinyl compounds such as. B. acrylonitrile and vinyl acetate copolymers.
3-sulfolene as a recyclable solvent
The reversibility of the formation of 3-sulfolene and its decomposition into the starting materials 1,3-butadiene and sulfur dioxide suggest that butadiene sulfone is used as a recyclable aprotic dipolar solvent and the similar, frequently used, but difficult to separate and poorly reusable dimethyl sulfoxide (DMSO) to replace. As a model reaction, the implementation of benzyl halides, such as. B. Benzyl chloride or benzyl bromide with sodium azide to benzyl azide and its reaction with 4-toluenesulfonic acid cyanide to 1-benzyl-5- (4-toluenesulfonyl) tetrazole used.
The synthesis can also be carried out as a one-pot reaction without isolation of the benzyl azide with a total yield of 72%. After the end of the reaction, the solvent butadiene sulfone is cleaved at 135 ° C and the volatile butadiene (m.p. −4.5 ° C) and sulfur dioxide (m.p. −10.05 ° C) are liquid in a cold trap filled with excess sulfur dioxide at −76 ° C deposited. When heated to room temperature - after the addition of hydroquinone to inhibit polymerization - 3-sulfolene is formed again practically quantitatively.
It seems questionable whether 3-sulfolene with a usable liquid phase range of 64 to a maximum of approx. 100 ° C as a DMSO substitute would have the "clear" advantages postulated from the 20 milliliter batches (easy handling, low costs, environmental compatibility) in industrial applications Can prove in practice.
Individual evidence
- ↑ a b c d Data sheet 3-Sulfolene, 98% from AlfaAesar, accessed on July 5, 2017 ( PDF )(JavaScript required) .
- ↑ a b c data sheet 3-Sulfolen at Sigma-Aldrich , accessed on July 55, 2017 ( PDF ).
- ↑ a b c d Data sheet 3-sulfolene for synthesis (PDF) from Merck , accessed on July 5, 2017.
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2017, ISBN 978-1-4987-5429-3 , pp. 3-188 .
- ↑ a b M.E. Brant, JE Wulff: 3-Sulfolenes and their derivatives: Synthesis and applications . In: Synthesis . tape 48 , no. 01 , 2016, p. 1-17 , doi : 10.1055 / s-0035-1560351 .
- ↑ a b c J.M. McIntosh: 3-sulfolenes . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2001, doi : 10.1002 / 047084289X.rs130 .
- ↑ a b c d Patent DE506839 : Process for the preparation of monomolecular reaction products of unsaturated hydrocarbons of the butadiene series with sulfur dioxide. Published on August 28, 1930 , applicant: H. Staudinger, inventor: H. Staudinger.
- ^ Houben-Weyl: Methods of Organic Chemistry, 4th Edition, Volume IX: Sulfur, Selenium, Tellurium Compounds . Georg Thieme Verlag, Stuttgart 1955, ISBN 978-3-13-208104-8 , p. 237 .
- ↑ J. Leonard, AB Hague, JA Knight: Organosulfur Chemistry, 4th Edition, Volume 2, 6. Preparation of substituted 3-sulfolenes and their use as precursors for Diels-Alder dienes . Academic Press, Inc., San Diego 1998, ISBN 0-12-543562-2 , pp. 241 .
- ↑ a b Patent US4187231 : Cyanide-catalyzed isomerization and deuterium exchange with alpha- and beta-sulfolenes. Filed November 16, 1977 , published February 5, 1980 , Applicant: Phillips Petroleum Co., Inventor: RL Cobb.
- ^ CD Broaddus: Equilibria and base-catalyzed exchange of substituted olefins . In: Acc. Chem. Res. Volume 1 , no. 8 , 1968, p. 231-238 , doi : 10.1021 / ar50008a002 .
- ↑ a b W.J. Bailey, EW Cummins: Cyclic dienes. III. The synthesis of thiophene-1,1-dioxide . In: J. Am. Chem. Soc. tape 76 , no. 7 , 1954, pp. 1932–1936 , doi : 10.1021 / ja01636a058 .
- ↑ a b H. Staudinger, B. Ritzenthaler: About high molecular compounds, 104th communication: About the addition of sulfur dioxide to ethylene derivatives . In: Chem. Ber. tape 68 , no. 3 , 1935, pp. 455-471 , doi : 10.1002 / cber.19350680317 .
- ↑ Patent US4286099 : Sulfolene hydrogenation. Filed February 12, 1980 , published August 25, 1981 , Applicant: Phillips Petroleum Co., Inventor: ME Nash, EE Huxley.
- ↑ a b D.M. Lemal, GD Goldman: Synthesis of azulene, a blue hydrocarbon . In: J. Chem. Educ. tape 65 , no. 10 , 1988, pp. 923 , doi : 10.1021 / ed065p923 .
- ↑ T.-S. Chou, M.-M. Chen: Chemoselective reactions of ultrasonically dispersed potassium with some brominated hydrothiophene-S, S-dioxides . In: Heterocycles . tape 26 , no. 11 , 1987, pp. 2829-2834 , doi : 10.3987 / R-1987-11-2829 .
- ↑ K. Hafner, KH Vöpel, G. Ploss, C. König: 6- (Dimethylamino) fulvene In: Organic Syntheses . 47, 1967, p. 52, doi : 10.15227 / orgsyn.047.0052 ; Coll. Vol. 5, 1973, p. 431 ( PDF ).
- ↑ D. Copland, D. Leaver, WB Menzies: A new and convenient synthesis of azulenes from 6-N, N-dimethylaminofulvene and thiophene-1,1-dioxides . In: Tetrahedron Lett. tape 18 , no. 7 , 1977, pp. 639-640 , doi : 10.1016 / S0040-4039 (01) 92713-3 .
- ↑ GS Andrade et al .: The one-pot synthesis and Diels-Alder reactivity of 2,5-dihydrothiophene-1,1-dioxide-3carboxylic acid . In: Synth. Commun. tape 33 , no. 20 , 2003, p. 3643-3650 , doi : 10.1081 / SCC-120024845 .
- ↑ K. Alder, HF Rickert, E. Windemuth: On the knowledge of the diene synthesis, X. Communication: About the diene synthesis with α, β- unsaturated nitro bodies, sulfones and thio ethers . In: Chem. Ber. tape 71 , no. 12 , 1938, pp. 2451-2461 , doi : 10.1002 / cber.19380711206 .
- ↑ TE Sample, Jr., LF Hatch: 3-Sulfolene: a butadiene source for a Diels-Alder synthesis: an undergraduate laboratory experiment . In: J. Chem. Educ. tape 45 , no. 1 , 1968, p. 55 , doi : 10.1021 / ed045p55 .
- ↑ TE Sample, Jr., LF Hatch: Diethyl trans-Δ 4 -tetrahydrophthalate In: Organic Syntheses . 50, 1970, p. 43, doi : 10.15227 / orgsyn.050.0043 ; Coll. Vol. 6, 1988, p. 454 ( PDF ).
- ↑ H. Hart, A. Bashir-Hashemi, J. Luo, MA Meador: Iptycenes: Extended triptycenes . In: Tetrahedron . tape 42 , no. 6 , 1986, pp. 1641-1654 , doi : 10.1016 / S0040-4020 (01) 87581-5 .
- ↑ C.-T. Lin, T.-C. Chou: Synthesis of 2,3-dibromoanthracene . In: Synthesis . tape 1988 , no. 8 , 1988, pp. 628-630 , doi : 10.1055 / s-1988-27659 .
- ↑ MP Cava, JP VanMeter: Condensed cyclobutane aromatic compounds. XXX. Synthesis of some unusual 2,3-naphthoquinonoid heterocycles. A synthetic route to derivatives of naphtho [2,3-b] biphenylene and anthra [b] cyclobutene . In: J. Org. Chem. Band 34 , no. 3 , 1969, p. 538-545 , doi : 10.1021 / jo01255a012 .
- ^ LF Hatch, D. Peter: Reaction of benzyne with butadiene . In: Chem. Commun. (London) . tape 23 , 1968, p. 1499 , doi : 10.1039 / C19680001499 .
- ↑ EJ Goethals: On the polymerization and copolymerization of sulfolenes . In: Macromol. Chem. Phys. tape 109 , no. 1 , 1967, p. 132-142 , doi : 10.1002 / macp.1967.021090113 .
- ↑ Y. Huang et al .: Butadiene sulfone as 'volatile', recyclable dipolar, aprotic solvent for conducting substitution and cycloaddition reactions . In: Sus. Chem. Proc. tape 3 , no. 13 , 2015, doi : 10.1186 / s40508-015-0040-7 .