2,3-dichloro-5,6-dicyano-1,4-benzoquinone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,3-dichloro-5,6-dicyano-1,4-benzoquinone | |||||||||||||||
| other names |
DDQ |
|||||||||||||||
| Molecular formula | C 8 Cl 2 N 2 O 2 | |||||||||||||||
| Brief description |
yellow to orange powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 227.00 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
210-217 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ from English 2,3-dichloro-5,6-dicyano-1,4-benzoquinone) is an organic compound and is used as an oxidative reagent in of organic chemistry its application. DDQ is used for the oxidative dehydrogenation of alcohols , phenols and steroid ketones . DDQ is also used in protecting group technology in the cleavage of p- methoxy and 3,4-dimethoxybenzyl ethers. DDQ decomposes in water, but is stable in the presence of aqueous mineral acids.
synthesis
In 1906 Johannes Thiele and Fritz Günther published a reaction sequence consisting of cyanation and chlorination of 1,4-benzoquinone . A one-step reaction for the synthesis of DDQ was reported in 1965 by Derek Walker and Thomas D. Waugh starting from 2,3-dicyanohydroquinone .
stability
DDQ is hydrolyzed by water, splitting off the very toxic hydrocyanic acid (HCN). Low temperatures and a weakly acidic environment increase the stability.
use
DDQ is a reagent that is used for oxidation and as a radical acceptor.
Reactions
Dehydrogenation
DDQ can be used for the dehydrogenation of α, β-unsaturated carbonyl compounds, as shown in the example of a steroid.
Flavoring
In the presence of acids, aromatic steroids are accessible by dehydration.
Oxidative coupling reactions
DDQ can also be used for oxidative couplings.
Individual evidence
- ↑ a b c d e f data sheet 2,3-dichloro-5,6-dicyano-1,4-benzoquinone from Acros, accessed on January 1, 2012.
- ↑ a b Braude. EA, Linstead, RP, and Wooldridge, KRH: Hydrogen Transfer. 9. The selective dehydrogenation of unsaturated alcohols by high-potential quinones . In: Journal of the American Chemical Society . August 1956, pp. 3070-3074. doi : 10.1039 / JR9560003070 .
- ↑ Becker. HD ,: Quinone Dehydrogenation .I. Oxidation Of Monohydric Phenols . In: Journal of Organic Chemistry . 30, No. 4, 1965, pp. 982-989. doi : 10.1021 / jo01015a006 .
- ^ AB Turner, HJ Ringold: Applications of high-potential quinones. Part I. The mechanism of dehydrogenation of steroidal ketones by 2,3-dichloro-5,6-dicyanobenzoquinone . In: Journal of the Chemical Society . 1967, pp. 1720-1730. doi : 10.1039 / J39670001720 .
- ↑ Yuji Oikawa, Tadao Yoshioka, Osamu Yonemitsu: "Specific removal of o-methoxybenzyl protection by DDQ oxidation", in: Tetrahedron Lett. , 1982 , 23 , pp. 885-888; doi : 10.1016 / S0040-4039 (00) 86974-9 .
- ↑ Johannes Thiele, Fritz Günther: About derivatives of Dicyanhydrochinons , in: Justus Liebigs Annalen der Chemie , 1906, Volume 349, Issue 1, pp. 45-66; doi : 10.1002 / jlac.19063490103 .
- ↑ Brown.W, Turner. AB, AB Turner: Application of High-potential Quinones. 7. Synthesis of steroidal phenanthrenes by double methyl migration . In: J. Chem. Soc. C. . No. 14, 1971, pp. 2566-2572. doi : 10.1039 / J39710002566 .
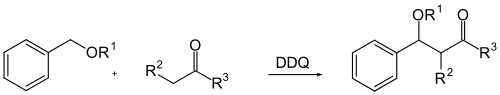
- ↑ YH.Zhang, CJ. Li, and Wooldridge, KRH: DDQ-Mediated Direct Cross-Dehydrogenative-Coupling (CDC) between Benzyl Ethers and Simple Ketones . In: Journal of the American Chemical Society . 128, No. 13, 2006, pp. 4242-4243. doi : 10.1021 / ja060050p .
Web links
- "Like Neurons in the Brain": A Molecular Computer that Evolves ( Memento from December 12, 2010 in the Internet Archive )