Diazomethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
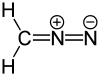
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diazomethane | |||||||||||||||
| other names |
Azimethylene |
|||||||||||||||
| Molecular formula | CH 2 N 2 | |||||||||||||||
| Brief description |
yellow gas smelling of damp foliage |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 42.04 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
1.45 g cm −3 |
|||||||||||||||
| Melting point |
−145 ° C |
|||||||||||||||
| boiling point |
−23 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 0.2 ml m −3 or 0.35 mg m −3 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Diazomethane ( empirical formula CH 2 N 2 ), also called azimethylene , is the simplest diazo compound . It is a methylating agent often used in the laboratory and is used in particular for the production of methyl esters from carboxylic acids and of cyclopropanes from alkenes. Its technical application is very limited due to its high toxicity and reactivity.
presentation

Diazomethane can be obtained by reaction of N -methyl-nitroso compounds such as N -methyl- N -nitrosoharnstoff or N -methyl- N -nitroso- p toluenesulfonamide with bases such as sodium or potassium hydroxide in diethylether prepared. A diazotate is initially formed, which is decomposed by the base in diazomethane and water. The diazomethane is distilled off with the ether. Another proposed mechanism describes a 1,3-acyl shift with a base-catalyzed cleavage.
properties
The molecular structure of diazomethane can be described by three mesomeric boundary structures:
The isomeric ring-shaped compound diazirine , whose structure was initially assigned to diazomethane, has completely different physical and chemical properties. Other isomeric forms of diazomethane are isopnallic acid amide (H 2 N-NC) and isodiazomethane (HCNNH), which are only stable in a complex-stabilized state.
At room temperature , diazomethane is a yellow gas that smells like damp leaves. It is extremely explosive both as a gas and as a liquid. That is why it is usually produced and used as a solution in diethyl ether. The ethereal solution is not stable, the diazomethane slowly decomposes with the release of nitrogen. There is a particular risk of explosion when there is contact with rough glass surfaces and metals. Diazomethane reacts explosively with alkali metals , calcium chloride , copper powder and sodium sulfide .
Its chemically characteristic reaction is methylation , in which diazomethane reacts with suitable nucleophiles , releasing nitrogen .
toxicity
Diazomethane is poisonous and carcinogenic in animal experiments . The main route of exposure for diazomethane is through the airways, where it reacts directly with tissue. Acutely it causes chemical burns, but little is known about its chronic toxicity.
use
Diazomethane is a powerful methylating agent and is only used as a solution in diethyl ether on a smaller scale. It is a very strong electrophile and has a good leaving group with N 2 . It is particularly suitable for the simple and effective production of methyl esters of carboxylic acids . In contrast to other methylations, methylation is also possible in weakly acidic conditions. Furthermore, it can be used for ring extensions , the Arndt-Eistert homologation for the extension of carbon chains by an additional carbon atom and [1,3] -dipolar cycloadditions .
If boron trifluoride is used as a catalyst , alcoholic hydroxyl groups (-OH) can be converted into methoxy groups (-OCH 3 ) with diazomethane . In contrast, phenols react with diazomethane even without a boron trifluoride catalyst.
The addition of diazomethane to C = C double bonds , for example in acrylonitrile , leads to the formation of pyrazoline rings .
Individual evidence
- ↑ a b c d e f g h i Entry on diazomethane in the GESTIS substance database of the IFA , accessed on February 18, 2017(JavaScript required) .
- ^ Hans Beyer, Wolfgang Walter, Wittko Francke: Textbook of organic chemistry . 23rd edition, S. Hirzel Verlag 1998, ISBN 3-7776-0808-4 .
- ↑ Entry on Diazomethane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 334-88-3 or diazomethane ), accessed on November 2, 2015.
- ^ A b Klaus Forstinger, Hans Joachim Metz: Diazo Compounds and Diazo Reactions. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2005, doi : 10.1002 / 14356007.a08_505 .
- ^ A b Arnold Willmes: Pocket book chemical substances: elements - inorganics - organic substances - natural substances - polymers . 3rd edition, Harri Deutsch Verlag, 2007, ISBN 978-3-8171-1787-1 , pp. 379-382.
- ↑ F. Arndt: Diazomethane In: Organic Syntheses . 15, 1935, p. 3, doi : 10.15227 / orgsyn.015.0003 ; Coll. Vol. 2, 1943, p. 156 ( PDF ).
- ↑ James A. Moore, Donald E. Reed: Diazomethane In: Organic Syntheses . 41, 1961, p. 16, doi : 10.15227 / orgsyn.041.0016 ; Coll. Vol. 5, 1973, p. 351 ( PDF ).
- ↑ Eberhard Breitmaier, Günther Jung Organic Chemistry Chapter 23.1 Vol. 7 Thieme 2012.
- ↑ Michael B. Smith, Jerry March. March's advanced organic chemistry sections, mechanisms and structure. Chapter 17 vol. 6 Wiley
- ↑ Diazirine. In: Römpp Chemistry Lexicon . Georg Thieme Verlag as of March 2002.
- ↑ Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 97.
- ↑ Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 607.
- ↑ Reinhard Brückner : reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 351.
- ↑ Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 668.
- ↑ Adalbert Wollrab: Organic Chemistry: An Introduction for Teaching and Minor Students. 3. Edition. Springer, 2009, ISBN 978-3-642-00780-4 , p. 440.
- ↑ Hans Peter Latscha, Uli Kazmaier, Helmut Alfons Klein: Organische Chemie: Chemie-Basiswissen II, Volume 2. 6th Edition, Springer, 2008, ISBN 978-3-540-77106-7 , p. 83.



