N -methyl- N -nitrosourea
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | N -methyl- N -nitrosourea | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 5 N 3 O 2 | |||||||||||||||
| Brief description |
colorless to yellowish needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 103.08 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.58 g cm −3 |
|||||||||||||||
| Melting point |
123 ° C (decomposition) |
|||||||||||||||
| solubility |
14.4 g l −1 in water (24 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
N -Methyl- N -nitrosourea is an unstable N - nitroso derivative of urea , which belongs to the group of nitrosoureas .
Presentation and extraction
The compound is made up of urea, methylamine and sodium nitrite . First, N- methylurea is obtained in a substitution reaction . The target compound results from a subsequent acid nitrosation.
properties
N -Methyl- N -nitrosourea is a crystalline solid that tends to decompose spontaneously. This can occur even when the compound is stored at room temperature. The decomposition occurs as a self-accelerating reaction. Contact with alkali hydroxides, dichloromethane or friction with metal spatulas can also trigger decomposition. The decomposition products found were methyl isocyanate , nitrogen and water . Slow decomposition results in N , N , N -trimethylcyanuric acid .
use
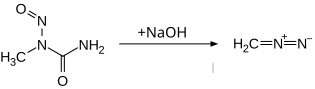
The compound can be used to make diazomethane . Because of the instability of the compound, however, other starting compounds such as N -methyl- N -nitroso- p -toluenesulfonamide , which are also not carcinogenic, are preferred today.
Individual evidence
- ↑ a b c d e f g Lutz Roth, Ursula Weller-Schäferbarthold: Hazardous chemical reactions. 69th supplementary delivery, ecomed-Verlag 2013.
- ↑ a b c d e Entry on N-methyl-N-nitrosourea in the GESTIS substance database of the IFA , accessed on March 23, 2018(JavaScript required) .
- ↑ F. Arndt: Nitrosomethylurea In: Organic Syntheses . 15, 1935, p. 48, doi : 10.15227 / orgsyn.015.0048 ; Coll. Vol. 2, 1943, p. 461 ( PDF ).
- ↑ F. Brogli, P. Grimm, M. Meyer, H. Zubler: Hazards of self-accelerating reactions. In: Swiss Chem. 3, 1981, 3a.
- ↑ F. Arndt: Diazomethan In: Organic Syntheses . 15, 1935, p. 3, doi : 10.15227 / orgsyn.015.0003 ; Coll. Vol. 2, 1943, p. 165 ( PDF ).