1,2,3-trihydroxybenzene
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1,2,3-trihydroxybenzene | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 6 O 3 | ||||||||||||||||||
| Brief description |
colorless, shiny leaves or needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 126.11 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.45 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
131-135 ° C |
||||||||||||||||||
| boiling point |
309 ° C |
||||||||||||||||||
| Vapor pressure |
2–4 hPa (140 ° C) |
||||||||||||||||||
| pK s value |
9.01 |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.561 (134 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
1,2,3-trihydroxybenzene (common name pyrogallol) is a derivative of benzene , a trivalent phenol with three vicinal hydroxyl groups. The other two isomers are 1,3,5-trihydroxybenzene (phloroglucinol) and 1,2,4-trihydroxybenzene (hydroxyhydroquinone).
history
1,2,3-trihydroxybenzene was produced in 1746 by Johann Heinrich Pott by subliming gall apples and first produced, characterized and named in 1786 by Carl Wilhelm Scheele by heating gallic acid . The common name pyrogallol is derived from the Greek pyr = fire and gallol from gallic acid; -ol refers to the hydroxyl (OH) groups and indicates the method of manufacture.
Extraction / representation
1,2,3-Trihydroxybenzene can most easily be produced by pyrolysis of gallic acid with decarboxylation .
It can also be prepared by hydrolysis of 2,2,6,6-tetrachlorocyclohexanone and oxidation of resorcinol with hydrogen peroxide . It is also created when tannins are broken down .
properties
In its pure state, 1,2,3-trihydroxybenzene consists of white crystal needles which, however, quickly turn gray-brown in color when exposed to air. It is a strong reducing agent and avidly absorbs oxygen from the air in alkaline solution ; it complexes metal ions (e.g. Fe 3+ with a blue color). When standing in the air for a long time (especially in an alkaline solution), 1,2,3-trihydroxybenzene is oxidized into carbon dioxide , acetic acid , purpurogalline (trihydroxybenztropolone) and other decomposition products; the solution then turns dark brown. As a result of this decomposition, the aqueous solutions gradually react more acidic, hence the name pyrogallic acid, which is not an appropriate name for the pure substance.
use
1,2,3-Trihydroxybenzene was previously used externally for psoriasis and lupus erythematosus . In analytical chemistry, 1,2,3-trihydroxybenzene is used to absorb oxygen in gas analysis , as a reagent for antimony , bismuth , niobium , osmium and tantalum , to reduce silver, gold and mercury salts and to determine the activity of peroxidases .
In microbiology , 1,2,3-trihydroxybenzene is used as a component of the pyrogallol seal ("Wright-Burri seal") in the cultivation of anaerobic bacteria . The absorption of oxygen from an alkaline 1,2,3-trihydroxybenzene solution in an airtight vessel is used (mostly with the help of a saturated calcium carbonate solution ). For this purpose, the 1,2,3-trihydroxybenzene-calcium carbonate mixture pipetted onto a suitable carrier material (e.g. cotton wool ) is applied over the nutrient medium .
It is also used in hair dye and is one of the oldest photographic developers and is still used today in holography . 1,2,3-Trihydroxybenzene is also used in lithography, in photoresists, in coating materials and in adhesives. It also serves as a crosslinker and hardener for epoxy resins , especially for the manufacture of electronic components. The compound is also used as an intermediate in the manufacture of various organic compounds such as pharmaceuticals. In addition to BHT , 1,2,3-trihydroxybenzene is sometimes used as a stabilizer for substances such as diethyl ether or carotenoids , which are subject to autoxidation by atmospheric oxygen.
proof
For qualitative and analytical proof, bromination with potassium bromide and bromine produces the dibromo derivative, which has a melting point of 158 ° C.
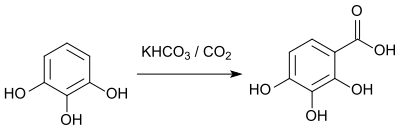
Reactions
The carboxylation of 1,2,3-trihydroxybenzene by a Kolbe-Schmitt reaction leads to 2,3,4-trihydroxybenzoic acid.
safety instructions
1,2,3-trihydroxybenzene is harmful to health. It is also gradually absorbed through the skin. In animal studies , as well as the attempt on human cells changes were the DNA to be detected.
Individual evidence
- ↑ a b c d e f g h i j k l m entry on pyrogallol. In: Römpp Online . Georg Thieme Verlag, accessed on August 2, 2018.
- ↑ a b c d e f Entry on 1,2,3-trihydroxybenzene in the GESTIS substance database of the IFA , accessed on January 23, 2020(JavaScript required) .
- ↑ CRC Handbook of Tables for Organic Compound Identification . 3. Edition. 1984, ISBN 0-8493-0303-6 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-36.
- ↑ Entry on pyrogallol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet 1,2,3-trihydroxybenzene (PDF) from Merck , accessed on January 18, 2011.
- ^ Pötsch et al: Lexicon of important chemists. 1989, p. 349, article Pott.
- ↑ Pamphlet on Biology: Kofoid collection . 1862, p. 61 ( limited preview in Google Book search).
- ↑ Nicole Borth, Brigitte Gasser, Regina Grillari, Renate Kunert, Ksenija Lopandic, Brigitte Lang, Diethard Mattanovich, Jutta Mattanovich, Christine Prenner, Beatrix Mayer-Reinprecht, Michael Sauer, Karola Vorauer-Uhl: work protocols 2018/19, 791.128 general microbiology exercises . Ed .: University of Natural Resources and Life Sciences, Vienna. Vienna 2018, p. 36 f .
- ^ Graham Saxby: Practical Holography, Third Edition . CRC Press, 2010, ISBN 1-4200-3366-2 , pp. 63 ( limited preview in Google Book search).
- ^ Joint FAO / WHO Expert Committee on Food Additives. Meeting: Compendium of Food Additive Specifications: Addendum 8 . Food & Agriculture Org., 2000, ISBN 92-5104508-9 , pp. 53 ( limited preview in Google Book search).
- ^ Mark S. Meskin, Wayne R. Bidlack, R. Keith Randolph: Phytochemicals: Aging and Health . CRC Press, 2008, ISBN 978-1-4200-6138-3 , pp. 68 ( limited preview in Google Book search).
- ^ Association of authors: Organikum . 19th edition. Johann Ambrosius Barth, Leipzig / Berlin / Heidelberg 1993, ISBN 3-335-00343-8 , p. 331.
- ^ Association of authors: Organikum . 19th edition. Johann Ambrosius Barth, Leipzig / Berlin / Heidelberg 1993, ISBN 3-335-00343-8 , p. 653.
- ^ VK Ahluwalia: Intermediates For Organic Synthesis . IK International Pvt Ltd, 2005, ISBN 81-88237-33-7 , pp. 86 ( limited preview in Google Book search).