Orsat apparatus
An Orsat apparatus is a historical laboratory device for volumetric gas analysis using chemical reactions .
It was patented by H. Orsat (i.e. Louis Orsat) in Paris in 1874. Today, however, this method is meaningless, since gas mixtures can be examined more precisely and elegantly with the help of gas chromatography .
Applications
This method was used to examine blast furnace top gas , generator gas , coke oven gas and exhaust gases in various industrial areas such as B. the burning of lime and cement .
construction
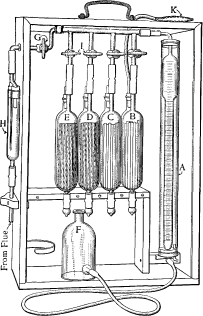
The Orsat apparatus consists of a gas burette A , which is provided with a temperature control jacket (thermostated water jacket) to keep the temperature constant. The gas mixture to be examined is recorded there. In addition, the Orsat apparatus consists of several special gas pipettes B - E , so-called. Orsat pipettes, the number of which varies depending on the gas mixture to be analyzed. The Orsat apparatus often also contains an incinerator (not shown) to determine the content of non-absorbable gases - such as hydrogen, methane, ethane, etc. - in the gas mixture. The gas burette, gas pipette and incinerator are connected to one another by individually lockable ( G and I ) capillary tubes . There is also the Orsat apparatus of a level bottle F .
function
The individual gas pipettes are filled with different liquids, e.g. B. so
- Pipette B with 25% potassium hydroxide solution to absorb carbon dioxide
- Pipette C with bromine water or 94% sulfuric acid and 1% silver sulfate for the absorption of alkenes
- Pipette D with pyrogallol solution to absorb oxygen
- Pipette E with ammoniacal copper (I) chloride solution to absorb carbon monoxide
The gas burette A is filled with sealing liquid ( aqueous solution mixed with sodium chloride or sulphate , often colored e.g. with methyl red ) and connected at the lower end to a level bottle via a hose. A measured volume of gas (e.g. 100 ml) is introduced into the burette via the upper stopcock G and the filter H , the sealing liquid being pressed into the equalization vessel. One after the other, the gas volume is passed through the individual gas pipettes by raising and lowering the expansion tank and opening the corresponding taps I. This absorbs the various gases one after the other. After each absorption step, the remaining volume of the gas in the gas burette is read off. An exact reading is achieved by bringing the liquid level of the sealing liquid in the level bottle to the same level as in the gas burette (principle of communicating tubes ). The difference to the initial volume (or the previous step) gives the volume fraction of the gases in the mixture. The volume of gas remaining after the last absorption step is assumed to be nitrogen .
Chemical reaction
Absorption of carbon dioxide
CO 2 + 2 KOH → K 2 CO 3 + H 2 O
Alkenes absorption
Example ethene in conc. Sulfuric acid>:
Absorption of oxygen
Absorption of carbon monoxide
CO + CuCl → [CuCl (CO) (H 2 O) 2 ]