Triethyl orthoformate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triethyl orthoformate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 16 O 3 | |||||||||||||||
| Brief description |
Flammable, moisture-sensitive, colorless liquid with an aromatic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 148.20 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.895 g cm −3 |
|||||||||||||||
| Melting point |
−76.1 ° C |
|||||||||||||||
| boiling point |
146 ° C |
|||||||||||||||
| Vapor pressure |
4 hPa (20 ° C) |
|||||||||||||||
| solubility |
Slightly soluble in water (1.35 g l −1 |
|||||||||||||||
| Refractive index |
1.3922 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Triethyl orthoformate is a chemical compound from the group of orthoesters . It is an ester of the hypothetical orthoformic acid HC (OH) 3 .
Extraction and presentation
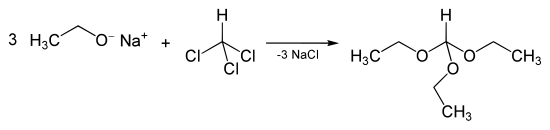
In addition to the esterification of formic acid, triethyl orthoformate can be obtained by reacting sodium ethanolate and chloroform :
properties
Triethyl orthoformate is a flammable, moisture-sensitive, colorless liquid with an aromatic odor.
use
Triethyl orthoformate is part of the Bodroux-Chichibabin-aldehyde synthesis , for example:
safety instructions
Triethyl orthoformate forms highly flammable vapor-air mixtures. The compound has a flash point of 34 ° C. The explosion range is between 0.85% by volume (52 g / m 3 ) as the lower explosion limit (LEL) and 27.2% by volume (1676 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 188 ° C. The substance therefore falls into temperature class T4.
Related links
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on triethyl orthoformate in the GESTIS substance database of the IFA , accessed on February 2, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-498.
- ↑ WE Kaufmann and EE Dreger: Ethyl orthoformate In: Organic Syntheses . 5, 1925, p. 55, doi : 10.15227 / orgsyn.005.0055 ; Coll. Vol. 1, 1941, p. 258 ( PDF ).
- ^ G. Bryant Bachman: n-Hexaldehyde In: Organic Syntheses . 16, 1936, p. 41, doi : 10.15227 / orgsyn.016.0041 ; Coll. Vol. 2, 1943, p. 323 ( PDF ).
- ^ A b E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.