Bodroux-Tschitschibabin aldehyde synthesis
The Bodroux-Tschitschibabin aldehyde synthesis , named after the Russian chemist Alexei Evgenjewitsch Tschitschibabin and Fernand Bodroux , is a name reaction from the field of organic chemistry and was first described in 1904.
Overview reaction
Starting from a Grignard compound (R = alkyl or aryl), the Bodroux-Tschitschibabin aldehyde synthesis achieves an extension of the carbon chain. The carbon chain is supplemented by a formyl group:
Triethyl orthoformate is usually used as the C1 building block (see section reaction mechanism).
Reaction mechanism
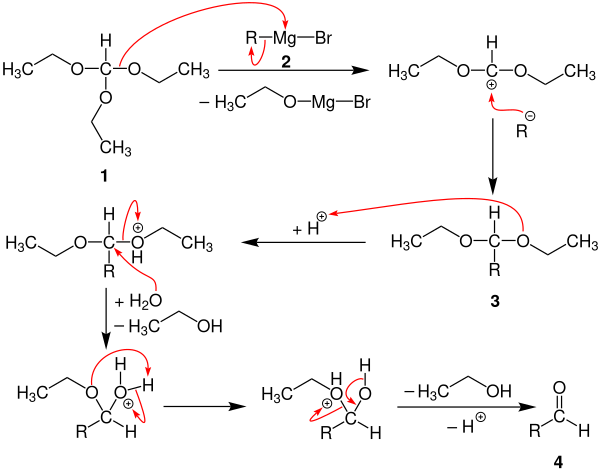
The following reaction mechanism is described in the literature:
Acetal 3 is initially formed by adding a Grignard compound 2 to triethyl orthoformate ( 1 ) . The aldehyde 4 is then synthesized by means of an acidic aqueous solution, with ethanol being eliminated twice . The reaction can also be carried out with other orthoformic acid esters.
Individual evidence
- ↑ a b AE Tschitschibabin: A new general method of representing aldehydes In: Ber. German Chem. Ges. 37, 1910, pp. 186-188, doi: 10.1002 / cber.19040370133 .
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents , Wiley, 2009, ISBN 978-0-471-70450-8 , pp. 448-451.
- ↑ MF Bodroux: Synthése d'aldehydes aromatiques In: Comptes Rendus Chimie . 138, 1904, pp. 92-94.