Organic light emitting diode
An organic light emitting diode ( English organic light emitting diode , OLED ) is a luminous thin film device of organic semiconducting material which is different from the inorganic light-emitting diodes is different (LED) that the electric current density and luminance are low and no single crystal materials are required. In comparison to conventional (inorganic) light-emitting diodes, organic light-emitting diodes can therefore be produced more cost-effectively using thin-film technology, but their service life and light yield are even lower than those of conventional light-emitting diodes.
The OLED technology is used for screens in smartphones , tablet computers as well as in large televisions and computer monitors. Another area of application is large-area room lighting . Due to the material properties, it is also possible to use the OLEDs as a flexible screen.
history
In the 1950s, electroluminescence in organic materials was discovered by A. Bernanose at the University of Nancy in France. Substances like acridine orange were deposited or dissolved in thin films made of cellulose or cellophane and exposed to an alternating current field . This mechanism is based on the direct excitation of dye molecules or electrons .
In 1960, Martin Pope and colleagues at New York University developed ohmic electrode contacts for injecting charge carriers into organic crystals when they were not illuminated. Furthermore, it describes the necessary energy requirements ( work function ) for electrode contacts, the electrons and holes ( positive holes ) in an organic semiconductor can inject. Such contacts are the basis for charge injection in all modern OLED devices.
In 1963, Pope's group also discovered the first direct voltage (DC) luminescence under vacuum on a pure anthracene single crystal and on tetracene -doped anthracene crystals with a small silver electrode at 400 V. This mechanism is based on field-accelerated electron excitation of molecular fluorescence . In 1965, Pope's group reported on the one hand electroluminescence in anthracene crystals, triggered by the recombination of thermalized electrons and holes without an external electric field, and on the other hand that the conductive energy level of anthracene is higher than the exciton energy level.
Also in 1965, Wolfgang Helfrich and WG Schneider of the National Research Council of Canada produced electroluminescence by double injected recombination for the first time in an anthracene single crystal using hole and electron injecting electrodes, the forerunners of modern double injecting devices.
In the same year researchers patented by Dow Chemical 1965, a process for the production of electroluminescent cells from an electrically insulated, 1 mm thin film of molten phosphorus with incorporated Anthracenpulver , tetracene and graphite powder , with the alternating voltage (100-3000 Hz , 500-1500 V ) was operated. This mechanism is based on the electronic excitation of graphite and anthracene molecules at the contacts.
The performance was limited by the poor electrical conductivity of the organic materials of the time. This limitation has been ameliorated with the discovery and development of highly conductive polymers . In 1975 , Roger Partridge from the British National Physical Laboratory first observed the electroluminescence of polymer films. The structure, which was later patented and published in a specialist journal in 1983, consisted of a film of polyvinyl carbazole up to 2.2 µm thick between two charge-injecting electrodes.
Ching W. Tang and Steven Van Slyke of the Eastman Kodak Company first reported a diode structure in 1987. A novel two-layer structure with a separate hole- and electron-transporting layer was used, so that recombination and light emission occurred in the middle of the organic layer. This led to a lower operating voltage and higher efficiency and marked the transition to today's OLED research and production.
In 1990, JH Burroughes and coworkers at the University of Cambridge developed an efficient green light emitting device using 100 nm thin film of poly (p-phenylene-vinylene) . In 1996, the first device with a luminous polymer was introduced by Cambridge Display Technology (CDT). In November 2006, scientists at the Pacific Northwest National Laboratory (PNNL) created a blue OLED with a quantum yield of 11% at 800 cd / m².
Structure and functionality
OLEDs are made up of several layers. Which is usually done to the anode consisting of indium tin oxide (ITO), which is located on a glass plate, a hole transport layer ( English hole transport layer , HTL applied). Depending on the manufacturing method, a layer of PEDOT / PSS is often applied between ITO and HTL , which serves to lower the injection barrier for holes and prevents indium from diffusing into the transition. A layer is applied to the HTL that either contains the organic dye (5 to 10 percent) or - rather rarely - consists entirely of the dye, e.g. B. aluminum tris (8-hydroxyquinoline) , Alq3. This layer is known as the emitter layer ( EL ). An electron transport layer ( ETL ) is optionally applied to this. Finally, a cathode consisting of a metal or an alloy with a low work function for electrons, such as calcium , aluminum , barium , ruthenium , magnesium-silver alloy, is vapor-deposited in a high vacuum. As a protective layer and to reduce the injection barrier for electrons, a very thin layer of lithium fluoride , cesium fluoride or silver is usually vapor-deposited between the cathode and E (T) L.
The electrons (i.e. the negative charge carriers) are now injected from the cathode, while the anode provides the holes (i.e. the positive charge carriers). Electrons and holes drift towards each other and ideally meet in the EL, which is why this layer is also known as the recombination layer . Electrons and holes form a bound state called an exciton . Depending on the mechanism, the exciton already represents the excited state of the dye molecule, or the decay of the exciton provides the energy to excite the dye molecule. This dye has different excited states . The excited state can change to the ground state and emit a photon ( light particle ). The color of the emitted light depends on the energy gap between the excited and ground state and can be changed in a targeted manner by varying the dye molecules. Non-radiating triplet states represent a problem. These can be resolved by adding so-called "excitors".
Use and selection of organic materials
The abbreviation PLED ( polymer light emitting diode ) has become established for organic LEDs made from polymers . As SOLED or SMOLED from "are rare small molecules " (small molecules ) called OLEDs manufactured. In PLEDs, derivatives of poly (p-phenylene-vinylene) (PPV) are often used as dyes . Recently, dye molecules have been used that are four times more efficient than the fluorescent molecules described above. In these more efficient OLEDs, metal-organic complexes are used in which the light is emitted from triplet states ( phosphorescence ).
These molecules are also called triplet emitters; the dye can also be excited by ambient light, which can lead to luminescence . The aim, however, is to produce self-illuminating screens that use organic electroluminescence .
In recent years, simulation techniques have been developed that can now calculate important properties of OLEDs completely on the computer based on the chemical composition. These methods allow an inexpensive pre-selection of molecules without complex synthesis and experimental characterization.
Manufacturing process for AMOLED screens
For AMOLED (active matrix OLED) screens there are two important manufacturing processes that determine the production costs, the technical properties and thus the field of application. On the one hand, there are RGB side-by-side AMOLED screens (SBS) based, among other things, on fine metal mask (FMM) technology and, on the other hand, white OLEDs with a color filter (WOLED).
RGB-SBS-AMOLED screens are designed in such a way that each subpixel emits one of the three primary colors red, green or blue. The advantages are a large color space (> 100% NTSC) and low power consumption compared to WOLED screens. However, the manufacturing process and the different rapid aging of the subpixels have a disadvantageous effect. During the manufacture of the SBS-AMOLEDs, the pixels are placed through a fine metal mask (FMM) or applied to the substrate. The problem here is the high accuracy with which the mask must be aligned (± 1 μm). Particularly with high resolutions and large screens, this often leads to manufacturing defects, resulting in high rejects and high costs. This technology is therefore used on small screens such as smartphones. Samsung is a big manufacturer here.
In contrast, WOLED technology does not produce different colored subpixels. A FMM is not used. With this technique, white emitted light for each subpixel hits a color filter that selects the basic colors per pixel. The lack of FMM means that large numbers of items can be produced even with large screens. Color shifts as a result of the emitter color layers aging at different rates, as in the case of SBS-AMOLED subpixels, do not occur here. However, burn-in effects are also possible with WOLEDs as with SBS-AMOLEDs due to different degrees of wear and tear on the subpixels, for example with the same image content. The power consumption of WOLEDs is higher because of the light-absorbing color filter and the color space can be smaller than that of SBS-AMOLEDs. LG, for example, uses WOLED technology in televisions.
advantages
One advantage of OLED screens compared to conventional liquid crystal screens (LCDs) is the very high contrast, as they do not have a backlight : Black pixels do not emit any light. While LCDs only act as colored filters and some light shines through in the dark, OLEDs only emit colored light when activated, which also promises very good color representation. This process is significantly more efficient, which means that OLEDs, especially when displaying dark images, require less energy. For this reason, OLED devices are less warm than corresponding devices with LC screens, although the conversion from cold cathode tubes to LEDs for LCD backlighting has reduced the energy consumption for liquid crystal screens. Due to their low energy consumption, OLEDs can be used in small, portable devices such as notebooks , cell phones and MP3 players . Because the background lighting is not required, it is possible to make OLEDs very thin. A Sony model presented at “Display 2008” has a depth of just 0.3 millimeters .
The response time (Engl. Response time ) of OLED screens located on some devices than 1 microsecond, making it about 1,000 times faster than the currently fastest LCD with a millisecond.
One advantage is based on the possibility of large-area production of OLEDs using printing technology , which does not require expensive vacuum and clean room conditions. The cost advantage results from the fact that the electrically conductive coloring layers can be applied in a modified inkjet printing process or, more recently, also in offset printing and can then be coated without vacuum deposition. DuPont and Merck are leaders in this area of soluble OLED material systems . The first OLEDs were printed under laboratory conditions as early as 1987 . The leading trade fair with congress for printed electronics is the annual LOPEC trade fair in Munich. At Drupa 2012, the leading trade fair for the printing industry, a. Printed OLEDs identified as a billion dollar market.
disadvantage
The biggest technical problem is the comparatively short service life of some components made of organic materials. In the case of (O) LEDs, the service life is the mean operating time after which the luminance has dropped by half. Blue OLEDs have the shortest lifespan. For white light sources, such as monitors, the blue component is therefore the limiting factor for the total useful life. In 2011, 5000 hours (at 1000 cd / m²) and 12,000 hours (at 100 cd / m²) were specified for white light sources. In comparison, standard white LEDs for the backlighting of LCD monitors have an average operating time on the order of 30,000 hours.
The service life of OLEDs depends on the temperature: A well-cooled OLED (regardless of the color) with a low initial luminosity always has a longer service life than an OLED that is operated with maximum luminosity from the start without cooling. The reason is the diffusion processes in the OLED, which run faster at higher temperatures. The service life at medium to low brightness is extrapolated from the value at maximum brightness , since testing OLED materials at low brightness for tens to a few hundreds of thousands of hours is not practical.
Another disadvantage of OLEDs is the lower luminous efficacy in the range of 40 lm / W to 60 lm / W compared to light-emitting diodes in commercially available OLEDs. Top values from selected laboratory samples for OLEDs achieve values just over 100 lm / W. Light-emitting diodes for lighting purposes achieve laboratory values of 200 lm / W.
In addition to a shorter service life and light yield, OLEDs are also sensitive to certain external substances. In addition to water, omnipresent air humidity , penetrating oxygen can also destroy the organic material. It is therefore important to hermetically encapsulate the display and protect it from external influences. The necessary rigid encapsulation impairs flexibility. Corrosion with oxygen primarily endangers the highly reactive injection layer made of calcium and barium . Typical signs of failure are circular, growing non-luminous areas, so-called “ dark spots ”. The cause is often a particle load when the metal layers are vapor deposited. The microscopic edges of the multilayer structure are also infiltrated by corrosion, which leads to a decrease in the effectively luminous pixel area in screen applications.
Commercial OLEDs on flexible substrates are in the introductory phase as of 2017, as all flexible plastic substrates have a high permeability for oxygen and air humidity. Very thin glass (glass with a maximum thickness of about 0.2 mm) is difficult to handle in processing, and the anode material indium tin oxide is a hard material and brittle. Repeated rolling in and out around a small radius leads to breakage and rapid failure (increase in resistance) of the anode.
State of the art
In many applications, OLEDs could replace the LCDs and plasma screens that are in use today . The lifespan still raises a few problems, because the red, green and blue luminous dots age at different rates. This irregular aging of the individual colors results in color shifts in the overall picture over time, which can only be compensated to a limited extent by - ideally automatic - readjustment (above all by increasing the blue emission).
The basic patents for OLED structures date from the 1980s. Here was Kodak leader. About 6,600 patents have been known on the subject since 1980. Research focuses on Japan , South Korea and the USA . Most of the patents are registered in Japan, followed by the USA and Europe. Germany ranks third with around 4.5%, behind the USA with around 22%.
Since self-illuminating OLED displays are even more expensive than backlit LC displays, they have so far only been used in special applications. Because of the smaller dimensions, they offer greater design freedom for the device manufacturer. The power consumption of OLEDs is also often lower since they do not require a backlight.
Large OLED screens have so far been more expensive than correspondingly large LCD screens. The main problems are the encapsulation of the components and the more complex control of the pixels. With LCDs, control takes place with low power, since LCD pixels as electrical capacitances are only reversed by an applied voltage, the light energy is generated by the backlight. In contrast, OLEDs themselves have to be supplied with the energy required to emit light in order to generate electroluminescence. They are current-controlled, which is why the mature technology used so far from the LCD field cannot be transferred directly.
In the case of small OLED screens, control can take place via a so-called passive matrix : A specific pixel is controlled by applying a voltage to a row and column, which requires two lines. This method is not sufficient for large screens because the web resistances increase linearly with the size and thus the driving force is no longer sufficient to control the respective pixel. To control the screen, an active matrix must be used in which each pixel is addressed individually via its own transistor, which requires four lines; derived from active matrix OLED ( active matrix organic light emitting diode ) the technology is sold under the terms AMOLED and SuperAMOLED . The provision of switching (voltage signals) as well as supply current is (as with plasma screens) complex and therefore expensive and one of the main reasons for the high costs of large screens.
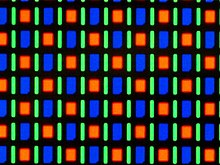
The latest technology is Super AMOLED + . The PenTile matrix has been removed here so that each pixel has all three basic colors available. Accordingly, without a pentile matrix, several pixels are no longer “connected” in order to mix all colors. As a result of this change, the resolution of such displays appears significantly higher, and no individual pixels stand out. Further improvements are better black values, increased contrast, more reproducible colors, lower power consumption and reduced thickness of the display. However, at very high pixel densities of well over 300 ppi ( pixels per inch ) , the PenTile effect is not noticeable or, if you look closely, is hardly noticeable. This is one of the reasons why Samsung can still use SuperAMOLED screens in newer products for Full HD without having to fear reduced quality.
Manufacturer
Major manufacturers of lamps using OLED technology are Konica-Minolta , OLEDWorks and Novaled GmbH, while LG , Samsung SDI and AU Optronics are important manufacturers of OLED information displays . There are also a number of other manufacturers. Philips and Osram left the display business in 2004 and 2007 and only produce OLED light sources. In mid-2015, Philips withdrew completely from OLED production and sold the production facility to OLED Works in Rochester, NY, USA.
In June 2015, the pharmaceutical and specialty chemicals manufacturer Merck KGaA laid the foundation stone for a new plant at its headquarters in Darmstadt, which will produce the basic chemical elements required for OLED technology for international OLED electronics manufacturers. The cost of the new factory was 30 million euros. The plant went into operation in September 2016.
In November 2015, the LG Group announced that it was building a plant for OLEDs in South Korea, which should start production in the first half of 2018. The investment costs are allegedly 8.2 billion euros.
During 2016, Samsung Electronics, a major manufacturer and user of AMOLEDs, announced that large TV screens would no longer be made using OLED technology in the future. In addition to limited service life, image burn-in phenomena and relatively high production costs are cited as reasons.
literature
- Dietmar Thomas: OLEDs: The new form of light. In: Dennis Köhler (Ed.): LED 2014 - Contributions to Technology , Highlight, 1st edition, Rüthen 2014, ISBN 978-3-937873-06-0 , pp. 217-225
- Chapter 3.11 OLED. In: Hans Rudolf Ris: Lighting technology for practitioners: Basics, lamps, lights, planning, measurement , VDE Verlag / Electrosuisse, 5th revised and expanded edition, Berlin / Offenbach 2015, ISBN 978-3-8007-3617-1 , p. 169-172
- Chapter 7 Organic Light Emitting Diodes (OLED). In: R. Heinz: Basics of light generation , 5th extended edition, Highlight, Rüthen, 2014, ISBN 978-3-937873-05-3 , pp. 115–126
- Joseph Shinar (Ed.): Organic Light-Emitting Devices: A Survey. Springer, New York 2004, ISBN 0-387-95343-4 .
- Hartmut Yersin (Ed.): Highly Efficient OLEDs with Phosphorescent Materials. Wiley-VCH, 2007, ISBN 3-527-40594-1 .
- WE Howard: Better displays with organic films. In: Scientific American . tape 290 , no. 2 , 2004, p. 76-81 , PMID 14743735 .
- Rick Li Zhigang: Organic Light-Emitting Materials and Devices . 2nd Edition. CRC Press, 2017, ISBN 978-1-138-74969-6 .
Web links
- OLED, LCD, TFT - structure and differences, advantages and disadvantages
- Organic light-emitting diodes - the wallpaper as a light source? ( Memento from June 5, 2009 in the Internet Archive )
- Pictures of OLED lights Examples of OLEDs in lighting with technical data
- Explanatory video for OLEDs on YouTube , accessed on October 6, 2018.
Individual evidence
- ↑ A. Bernanose, M. Comte, P. Vouaux: Sur un nouveau mode d'émission luraineuse chez certains composés organiques . In: J. Chim. Phys . tape 50 , 1953, pp. 64-68 .
- ↑ A. Bernanose, P. Vouaux: Electroluminescence organique: étude du mode d'emission . In: J. Chim. Phys . tape 50 , 1953, pp. 261 .
- ↑ A. Bernanose: Sur le mécanisme de l'électroluminescencc organique . In: J. Chim. Phys . tape 52 , 1955, pp. 396-400 .
- ↑ A. Bernanose, P. Vouaux: Relation entre l'électroluminescence organique et la concentration en produit actif . In: J. Chim. Phys . tape 52 , 1955, pp. 509 .
- ↑ H. Kallmann, M. Pope: Positive Hole Injection into Organic Crystals . In: The Journal of Chemical Physics . tape 32 , no. 1 , 1960, p. 300-301 , doi : 10.1063 / 1.1700925 .
- ↑ H. Kallmann, M. Pope: Bulk Conductivity in Organic Crystals . In: Nature . tape 186 , no. 4718 , 1960, pp. 31-33 , doi : 10.1038 / 186031a0 .
- ↑ Peter Mark, Wolfgang Helfrich: Space-Charge-Limited Currents in Organic Crystals . In: Journal of Applied Physics . tape 33 , no. 1 , 1962, pp. 205-215 , doi : 10.1063 / 1.1728487 .
- ↑ M. Pope, H. P. Kallmann, P. Magnante: Electroluminescence in Organic Crystals . In: The Journal of Chemical Physics . tape 38 , no. 8 , 1963, pp. 2042-2043 , doi : 10.1063 / 1.1733929 .
- ↑ Mizuka Sano, Martin Pope, Hartmut Kallmann: Electroluminescence and Band Gap in anthracenes . In: The Journal of Chemical Physics . tape 43 , no. 8 , 1965, p. 2920-2921 , doi : 10.1063 / 1.1697243 .
- ^ W. Helfrich, WG Schneider: Recombination Radiation in Anthracene Crystals . In: Physical Review Letters . tape 14 , no. 7 , 1965, pp. 229-231 , doi : 10.1103 / PhysRevLett.14.229 .
- ↑ Patent US3172862 : Organic electroluminescent phosphors. Published March 9, 1965 , Inventors: E. Gurnee, R. Fernandez.
- ↑ a b Letter OLED history . Comboled Project. Retrieved July 26, 2010.
- ↑ Patent US3995299 : Radiation sources. Published November 30, 1976 , inventor: Roger Hugh Partridge.
- ^ RH Partridge: Electroluminescence from polyvinylcarbazole films: 1. Carbazole cations . In: polymer . tape 24 , no. 6 , 1983, pp. 733-738 , doi : 10.1016 / 0032-3861 (83) 90012-5 .
- ↑ RH Partridge: Electroluminescence from polyvinylcarbazole films: 2. Polyvinylcarbazole films containing antimony pentachloride . In: polymer . tape 24 , no. 6 , 1983, pp. 739-747 , doi : 10.1016 / 0032-3861 (83) 90013-7 .
- ^ RH Partridge: Electroluminescence from polyvinylcarbazole films: 3. Electroluminescent devices . In: polymer . tape 24 , no. 6 , 1983, pp. 748-754 , doi : 10.1016 / 0032-3861 (83) 90014-9 .
- ^ RH Partridge: Electroluminescence from polyvinylcarbazole films: 4. Electroluminescence using higher work function cathodes . In: polymer . tape 24 , no. 6 , 1983, pp. 755-762 , doi : 10.1016 / 0032-3861 (83) 90015-0 .
- ↑ C. W Tang, S. A VanSlyke: Organic electroluminescent diodes . In: Applied Physics Letters . tape 51 , no. 12 , 1987, pp. 913-915 , doi : 10.1063 / 1.98799 .
- ↑ JH Burroughes, DDC Bradley, AR Brown, RN Marks, K. Mackay, RH Friend, PL Burns, AB Holmes: Light-emitting diodes based on conjugated polymers . In: Nature . tape 347 , no. 6293 , 1990, pp. 539-541 , doi : 10.1038 / 347539a0 .
- ↑ History of the company CDT (Cambridge Display Technology)
- ↑ "Record EQE in Blue OLED Device" in the Research Highlights 2005-2006 of the United States Department of Energy , January 6, 2012
- ↑ Detlef Mietke: Organic light-emitting diode - OLED. In: elektroniktutor.de. Retrieved July 20, 2016 .
- ↑ Christine Aust, Stefan Worlitzer: Functionality and properties of OLEDs. In: elektronikpraxis.vogel.de. September 7, 2006, accessed July 20, 2016 .
- ↑ Arndt Reuning : High luminosity without expensive metals in dradio “ Research News ” from November 27, 2013
- ↑ Hartmut Yersin: Triplet emitters for OLEDs. Introduction to exciton formation, charge transfer states, and triplet harvesting.
- ^ H. Yersin: Triplet Emitters for OLED Applications. Mechanisms of Exciton Trapping and Control of Emission Properties . In: Topics in Current Chemistry . tape 241 , 2004, pp. 1-26 , doi : 10.1007 / b83770 .
- ↑ Leni Akcelrud: Electroluminescent polymers . In: Progress in Polymer Science . tape 28 , no. 6 , 2003, p. 875-962 , doi : 10.1016 / S0079-6700 (02) 00140-5 .
- ↑ Max Planck Institute for Polymer Research: From Molecules to OLEDs.
- ↑ P. Kordt et al .: Modeling of Organic Light Emitting Diodes: From Molecular to Device Properties . In: Advanced Functional Materials . tape 25 , 2015, p. 1955–1971 , doi : 10.1002 / adfm.201403004 .
- ↑ Glory KJ Chen, Janglin Chen: AMOLED Manufacture . In: Handbook of Visual Display Technology . 2nd Edition. Springer-Verlag, Berlin / Heidelberg 2015, p. 1 .
- ↑ a b Glory KJ Chen, Janglin Chen: AMOLED Manufacture . In: Handbook of Visual Display Technology . 2nd Edition. Springer-Verlag, Berlin / Heidelberg 2015, p. 4 .
- ↑ a b Glory KJ Chen, Janglin Chen: AMOLED Manufacture . In: Handbook of Visual Display Technology . 2nd Edition. Springer-Verlag, Berlin / Heidelberg 2015, p. 4, 18 .
- ↑ a b Glory KJ Chen, Janglin Chen: AMOLED Manufacture . In: Handbook of Visual Display Technology . 2nd Edition. Springer-Verlag, Berlin / Heidelberg 2015, p. 5 .
- ↑ Large Screen OLED TVs Debut at CES | SEMI.ORG. Accessed June 2, 2017 .
- ↑ a b Glory KJ Chen, Janglin Chen: AMOLED Manufacture . In: Handbook of Visual Display Technology . 2nd Edition. Springer-Verlag, Berlin / Heidelberg 2015, p. 16-18 .
- ↑ Live long and phosphor: Blue LED breakthrough for efficient electronics. September 25, 2014. Retrieved March 16, 2019 (American English).
- ^ Karlheinz Blankenbach: Temporal Effects . In: Chen J., Cranton W., Fihn M. (Eds.): Handbook of Visual Display Technology . Springer, Berlin, Heidelberg 2015, ISBN 978-3-642-35947-7 .
- ↑ Large Screen OLED TVs Debut at CES | SEMI.ORG. Accessed June 2, 2017 .
- ↑ Jan Johannsen: 0.3 millimeters: Ultra-thin screen from Sony . On: www.netzwelt.de , April 18, 2008.
- ↑ Universal Display Corporation : OLED Marketplace . Retrieved September 21, 2007.
- ↑ Process reduces OLED production cost . Retrieved June 28, 2012.
- ↑ High-Performance DuPont OLED Materials . Retrieved June 28, 2012.
- ↑ OLED materials - for revolutionary high-performance displays . Retrieved June 28, 2012.
- ↑ LOPE-C (Large-area, Organic and Printed Electronics Convention) . Retrieved June 28, 2012.
- ↑ Visions come true: Conquering the markets with printed electronics ( Memento from April 5, 2016 in the Internet Archive ). Retrieved June 28, 2012.
- ↑ [1] October 1, 2010.
- ↑ [2] April 11, 2011.
- ↑ LED backlight LCD monitor, manufacturer datasheet. Retrieved January 1, 2018 .
- ↑ Manuel Boesing, et. al: Recent Advances in OLED Lighting. Accessed December 31, 2017 .
- ↑ List of OLED manufacturers .
- ↑ Osram pulls the rip cord in the paralyzing OLED display business . On: www.golem.de , July 31, 2007.
- ↑ Press release from Philips . On: www.philips.de , July 16, 2010.
- ↑ OLEDWorks to buy the key parts of Philips OLED light source components business . On: www.oledworks.com , April 28, 2015.
- ↑ Merck opens new OLED production facility in Darmstadt. (No longer available online.) In: merck.de. Archived from the original on January 6, 2016 ; accessed on September 7, 2016 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ LG is investing billions in new technology . In: handelszeitung , November 27, 2015. Accessed November 27, 2015.
- ↑ Lee Gijong: Samsung Electronics to skip OLED TV and go straight to QLED TV. iPnomics, May 24, 2016; accessed January 11, 2017.
- ↑ Wolfgang Tunze: All things that tick digitally. In: NZZ am Sonntag , January 8, 2017, p. 54.





