Phase transfer catalysis
The phase transfer catalysis (PTC) is a chemical process in which the reactants in at least two immiscible phases are present. A phase transfer catalyst enables one of the reactants to pass through the phase boundary into the phase in which the chemical process takes place.
General
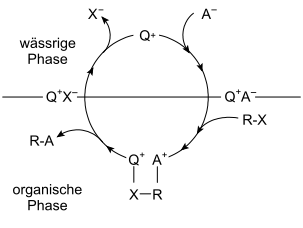
In practice, the phases are often water and an organic solvent. The transferred reactant is usually an ion that is normally not soluble in the organic phase and passes from the aqueous phase to the organic phase. Often transferred anions are e.g. B. the hydroxide ion , the cyanide ion or an oxidizing agent such as permanganate , but also cations can be transferred from the aqueous to the organic phase.
Quaternary ammonium salts are often used as phase transfer catalysts for anions ; the transfer of cations into an organic phase takes place e.g. B. with the help of crown ethers .
In inverse phase transfer catalysis, the substrate, for example carboxylic acid halides , is dragged into the aqueous phase by means of an inverse phase transfer catalyst, for example pyridine derivatives, and can react there.
Examples
Phase transfer catalysis (PTC) is applicable to many types of reactions. One example is nucleophilic substitution . A reaction of sodium cyanide present in the aqueous phase and an alkyl halide such as 1-bromooctane in the organic phase does not take place under non-catalyzed conditions. By adding phase transfer catalysts such as phosphonium salts (e.g. hexadecyltributylphosphonium bromide), cyanide ions are easily transferred into the organic phase, where they react quantitatively with the alkyl halide to form 1-cyanooctane .
One of the first examples of phase transfer catalysis was discovered by Mieczysław Mąkosza in 1960 and published in 1965. This involves an alkylation of phenylacetonitrile with the help of an alkyl chloride, a quaternary ammonium salt as a catalyst and an aqueous sodium hydroxide phase ( sodium hydroxide solution ):
Another example is the cycloaddition of dichlorocarbene generated in situ on a cyclohexene with the aid of phase transfer catalysis. This creates 7,7-dichloronorcaran:
Subsequent work by Herriott and Picker showed that many such reactions can be carried out at room temperature by using quaternary ammonium compounds in the water / benzene system . By using chiral phase transfer catalysts, a stereoselective effect can optionally be achieved in certain reactions .
With PTC, certain reactions can be carried out at higher conversion rates and yields with fewer by-products. Also, the need for expensive solvents that would dissolve both reactants can often be reduced and allows the use of inexpensive water and common organic solvents.
The use of PTC is not limited to the water / organic solvent system; it can also be used in solid / liquid or liquid / gas reactions.
Web links
- Examples of phase transfer catalysis (PDF file; 23 kB)
- Phosphonium salts as phase transfer catalysts and the use of the corresponding phosphines in asymmetric catalysis
Individual evidence
- ↑ Organikum, 22nd edition, chap. 3.2. Carboxylic acids from primary alcohols and olefins with phase transfer catalysis, p. 436.
- ^ Tamon Okano: Counter-phase Transfer Catalysis . In: Boy Cornils , Wolfgang A. Herrmann (Ed.): Aqueous-Phase Organometallic Catalysis: Concepts and Applications . John Wiley & Sons, 2004, ISBN 978-3-527-30712-8 ( limited preview in Google Book Search).
- ↑ Eckehard V. Dehmlow : Phase transfer-catalyzed two-phase reactions in preparative organic chemistry . In: Angewandte Chemie . tape 86 , no. 5 , 1974, p. 187-196 , doi : 10.1002 / anie.19740860503 .
- ^ EV Dehmlow and SS Dehmlow: Phase Transfer Catalysis. 3rd revised and expanded edition, VCH Verlagsgesellschaft, Weinheim / VCH Publishers, New York, 1993. 499 pp., ISBN 3-527-28408-7 .
- ^ Starks, CM: Phase-transfer catalysis . I. Heterogeneous reactions involving anion transfer by quaternary ammonium and phosphonium salts. In: J. Am. Chem. Soc. tape 93 , no. 1 , 1971, p. 195-199 , doi : 10.1021 / ja00730a033 .
- ↑ M. Makosza, A. Jonczyk: Phase-Transfer Alkylation of Nitriles: 2-Phenylbutryonitrile . In: Organic Syntheses . tape 55 , 1976, pp. 91 , doi : 10.15227 / orgsyn.055.0091 .
- ↑ Marek Chmielewski, Janusz Jurczak: A Tribute to Prof. Mieczyslaw Makosza. Commerative Issue in Honor of Prof. Mieczyslaw Makosza on the occasion of his 70th anniversary . tape 2004 , no. 3 , 2003, p. 1-4 , doi : 10.3998 / ark.5550190.0005.301 .
- ^ Lutz F. Tietze: Reactions and syntheses in the organic-chemical internship and research laboratory . 2nd Edition. Wiley-VCH, Weinheim 2004, ISBN 3-527-30874-1 , doi : 10.1002 / 3527601716 .
- ^ AW Herriott, D. Picker: Phase transfer catalysis. Evaluation of catalysis . In: J. Am. Chem. Soc. tape 97 , no. 9 , 1975, p. 2345-2349 , doi : 10.1021 / ja00842a006 .
- ↑ Jürgen O. Metzger: Solvent-Free Organic Syntheses . In: Angewandte Chemie International Edition . Wiley Online Library. tape 37 , no. 21 , November 16, 1998, pp. 2975-2978 , doi : 10.1002 / (SICI) 1521-3773 (19981116) 37:21 <2975 :: AID-ANIE2975> 3.0.CO; 2-A .
- ↑ M. Makosza: phase-transfer catalysis. A general green methodology in organic synthesis . In: Pure Appl. Chem. Band 72 , no. 7 , 2000, pp. 1399-1403 , doi : 10.1351 / pac200072071399 .