Milnacipran
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
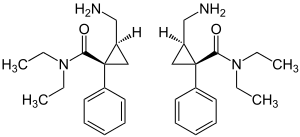
| 1: 1 mixture: (1 R , 2 S ) -isomer (left) and (1 S , 2 R ) -isomer (right) | |||||||||||||
| General | |||||||||||||
| Non-proprietary name | Milnacipran | ||||||||||||
| other names | |||||||||||||
| Molecular formula |
|
||||||||||||
| Brief description |
white powder (milnacipran hydrochloride) |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| Mechanism of action | |||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
179–181 ° C (Milnacipran hydrochloride) |
||||||||||||
| solubility |
Water: 19 g l −1 (HCl) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Milnacipran is a drug that has been approved as an antidepressant in some EU countries and is a selective serotonin-norepinephrine reuptake inhibitor (SSNRI).
Indications
In Austria it was approved as an antidepressant in 1998. In Germany, Milnacipran was approved in 2016. The manufacturer Pierre Fabre Médicament has not yet applied for approval in Switzerland . The active ingredient is approved as an antidepressant in over 40 countries, including France, Russia, Japan, Finland.
In the US Milnacipran is for the indication fibromyalgia - syndrome approved (FMS) since in 2009. In Europe, however, the approval for this was refused due to insignificant effects and a lack of long-term data in a European population .
In a 2008 meta-analysis it was found that there was no demonstrable difference in the effectiveness and tolerability of Milnacipran compared to other antidepressants, although it was found that fewer patients discontinued treatment due to side effects. A Cochrane review from 2009 concludes that there is a lack of information on clinically meaningful outcomes such as cost-effectiveness and ability to return to work.
pharmacology
Milnacipran inhibit the central nervous system to resume (engl .: reuptake ) of serotonin and norepinephrine in the synaptic cleft into presynaptic vesicles . The substance is pharmacologically assigned to the selective serotonin-noradrenaline reuptake inhibitors (SSNRI). This group also includes venlafaxine and duloxetine . While Milnacipran blocks serotonin and noradrenaline reuptake approximately equally, duloxetine has a 10-fold greater selectivity for serotonin, venlafaxine a 30-fold greater selectivity for serotonin. The substance has no affinity for postsynaptic receptors.
Contraindications (contraindications)
Milnacipran must not be used in the following cases:
- when using non-selective MAO-inhibitors and selective MAO-A-inhibitors: risk of serotonin syndrome
- selective MAO-B inhibitors, digitalis , 5-HT1D agonists (e.g. sumatriptan ), parenteral adrenaline and noradrenaline as well as clonidine and related substances: risk of hypertonic crises
- for benign prostatic hyperplasia and other urogenital disorders
- during the breastfeeding period.
The general warning issued by the European Medicines Agency for SSRIs and SNRIs regarding the therapy of children and adolescents must be observed.
Interactions with other drugs
Milnacipran is not degraded by liver cytochrome P450 and has no clinically significant interactions with any of the CYP systems. Milnacipran does not change the plasma levels of concurrent medications. There are also no known interactions with alcohol.
Pharmacokinetics
Milnacipran is well absorbed after oral administration. The bioavailability is around 85% and is not affected by food intake. The peak plasma concentration is reached after oral intake about two hours and is approx. 120 ng / ml after a single dose of 50 mg. The concentrations increase proportionally to the dose up to 200 mg per dose. The plasma protein binding is low (13%) and is not saturable. The volume of distribution of Milnacipran is approximately 5 L / kg with a total clearance of approximately 40 L / h. Renal and non-renal clearance are equivalent. The metabolism of milnacipran is limited mainly to a glucuronide conjugation. Very low levels of active metabolites with no clinical relevance have been demonstrated. The plasma half-life is about 8 hours. Excretion mainly takes place via the kidneys (90% of the administered dose) with tubular secretion of the unchanged product. After repeated dosing, Milnacipran is completely eliminated two to three days after stopping treatment. A liver failure caused no significant change in the pharmacokinetics of the drug. Milnacipran should be used with caution in patients with renal insufficiency - the dose may need to be reduced due to a prolonged elimination half-life .
Stereoisomerism
2- (Aminomethyl) - N , N -diethyl-1-phenyl cyclopropanecarbamide contains two stereocenters . Thus there are the following four forms:
- (1 R , 2 S ) - isomer
- (1 S , 2 R ) isomer
- (1 R , 2 R ) isomer
- (1 S , 2 S ) isomer
The drug Milnacipran is a racemate (1: 1 mixture) of the (1 R , 2 S ) isomer and the (1 S , 2 R ) isomer. The (1 R , 2 R ) -isomer and the (1 S , 2 S ) -isomer have no practical significance.
Trade names
Ixel (A), Savella (USA), Toledomin (Japan), Joncia (Australia), Tivanyl (Mexico), Dalcipran (Chile), Milna-neurax (D)
literature
- Effect of repeated treatment with milnacipran on the central dopaminergic system . In: Pol J Pharmacol . 2000 Mar-Apr; 52 (2): 83-92; PMID 10949109 .
- SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants . In: CNS Spectr . 2005 Sep; 10 (9): 732-747; PMID 16142213 .
Individual evidence
- ↑ a b c d Data sheet Milnacipran hydrochloride from Sigma-Aldrich , accessed on April 10, 2011 ( PDF ).
- ↑ Entry on Milnacipran. In: Römpp Online . Georg Thieme Verlag, accessed on November 12, 2014.
- ↑ a b The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 1069, ISBN 978-0-911910-00-1 .
- ↑ Questions and Answers on the Marketing Authorization Refusal Recommendation for Milnacipran Pierre Fabre Médicament / Impulsor. (41 kB; PDF) In: Doc.-Ref .: EMA / 64535/2010, EMEA / H / C / 1034, EMEA / H / C / 1122. Committee for Medicinal Products for Human Use (CHMP), February 3, 2010, p. 2 , accessed on August 24, 2010 : “Effect ... insignificant. ... long-term effects in a European population ... were missing. ... there are currently no clinical trials in Europe ... in fibromyalgia ” .
- ↑ REFUSAL ASSESSMENT REPORT FOR Milnacipran Pierre Fabre Medicament. (608 kB; PDF) In: Procedure No. EMEA / H / C / 001034. European Medicines Agency, April 26, 2010, accessed on August 24, 2010 (English, 50 pages).
- ↑ Nakagawa A, Watanabe N, Omori IM, et al. : Efficacy and tolerability of milnacipran in the treatment of major depression in comparison with other antidepressants: a systematic review and meta-analysis . In: CNS Drugs . 22, No. 7, 2008, pp. 587-602. PMID 18547127 .
- ↑ Nakagawa A, Watanabe N, Omori IM, et al .: Milnacipran versus other antidepressive agents for depression . In: Cochrane Database Syst Rev. . 8, No. 3, p. CD006529. PMID 19588396 .
- ↑ Moret C, Charveron M, Finberg JP, Couzinier JP, Briley M: Biochemical profile of midalcipran (F 2207), 1-phenyl-1-diethyl-aminocarbonyl-2-aminomethyl-cyclopropane (Z) hydrochloride, a potential fourth generation antidepressant drug . In: Neuropharmacology . 24, No. 12, 1985, pp. 1211-9. doi : 10.1016 / 0028-3908 (85) 90157-1 . PMID 3005901 .
- ↑ a b Christian Puozzo, Simone Lens, Christian Reh, Karl Michaelis, Dominique Rosillon, Xavier Deroubaix, Dominique Deprez: Lack of Interaction of milnacipran with the cytochrome P450 isoenzymes Frequently Involved in the Metabolism of Antidepressants . In: Clinical Pharmacokinetics . 44, No. 9, 2005, pp. 977-88. PMID 16122284 .
- ↑ Puozzo C, Albin H, Vinçon G, Deprez D, Raymond JM, Amouretti M: Pharmacokinetics of milnacipran in liver impairment . In: European Journal of Drug Metabolism and Pharmacokinetics . 23, No. 2, 1998, pp. 273-9. PMID 9725493 .
- ↑ Puozzo C, Pozet N, Deprez D, Baille P, Ung HL, Zech P: Pharmacokinetics of milnacipran in renal impairment . In: European Journal of Drug Metabolism and Pharmacokinetics . 23, No. 2, 1998, pp. 280-6. PMID 9725494 .
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Milnacipran
