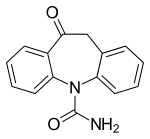
Oxcarbazepine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Oxcarbazepine | ||||||||||||||||||
| other names |
10-Oxo-10,11-dihydro-5 H -dibenzo [ b , f ] azepine-5-carboxamide |
||||||||||||||||||
| Molecular formula | C 15 H 12 N 2 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action |
Blockage of the sodium channels and ion channels |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 252.3 g · mol -1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Oxcarbazepine belongs chemically to the class of dibenzazepines and is a descendant of carbamazepine . It is a drug used as an anticonvulsant in the long-term treatment of certain forms of epilepsy .
pharmacology
Oxcarbazepine is metabolized to 10,11-dihydro-10-hydroxycarbamazepine, which is the actually active substance and which is determined in the blood for therapy control. High fluctuations are observed here in individual individuals. This different metabolic pathway means that there are fewer interactions with other drugs.
Range of applications
In accordance with the spectrum of activity of carbamazepine, oxcarbazepine is indicated for epilepsy with focal and secondary generalized seizures in adults and children. Oxcarbazepine is considered to be better tolerated than carbamazepine, but this has not been proven beyond doubt. Also due to this unclear data situation, carbamazepine is still being prescribed significantly more frequently than oxcarbazepine.
unwanted effects
The most common side effects include:
- Drowsiness (22.6%)
- Dizziness (22.6%)
- Drowsiness (22.5%)
- Headache (14.6%)
- Nausea (14.1%)
- Double vision (13.9%)
- Feeling weak (12%)
- Vomiting (11.1%)
There may be a slight transient increase in liver enzymes without clinical significance. Idiosyncratic / allergic (i.e. independent of the dose) , skin rashes can also occur less frequently than with carbamazepine . However, in around a quarter of all cases there is a so - called cross allergy . This means that patients who react to carbamazepine with a skin rash will also get it after being given oxcarbazepine. In 23% to 73% of users, long-term use leads to hyponatremia ( electrolyte shift with a reduction in the content of sodium ions in the blood ). Unlike valproate , which the thyroid gland does not affect, the examined men were found in 24% serum - thyroxine -mirror below the norm.
Interactions
Oxcarbazepine can make hormonal contraceptives ( birth control pills ) and some calcium channel blockers ineffective by affecting the enzymes in the cytochrome P450 complex in the liver and lowering the plasma levels of other drugs .
Effects on ability to drive and use machines
Oxcarbazepine can affect your ability to drive and operate machines.
Trade names
Apydan Extent (D, CH), Timox (D), Trileptal (D, A, CH), various generics (D, A)
Individual evidence
- ↑ a b Oxcarbazepine data sheet from Sigma-Aldrich , accessed on June 16, 2011 ( PDF ).
- ↑ Hermann Stefan: Epilepsy Therapy (PDF; 114 kB). In: Dtsch. Ärzteblatt 95, Issue 49, A-3128, December 4, 1998.
- ^ H. Siemes, BFD Bourgeois: Seizures and epilepsies in children and adolescents. Thieme, Stuttgart / New York 2001, p. 259.
- ^ MW Koch, SKL Polman: Oxcarbazepine versus carbamazepine monotherapy for partial onset seizures. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No .: CD006453. doi : 10.1002 / 14651858.CD006453.pub2 .
- ^ U. Schwabe, D. Paffrath: Drug Ordinance Report 2010. Springer Medizin Verlag, Heidelberg.
- ↑ a b c Specialist information of the Swiss Medicines Compendium: Trileptal.