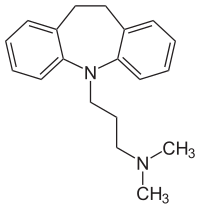
Imipramine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Imipramine | |||||||||||||||||||||
| other names |
10,11-dihydro- N , N -dimethyl-5 H -dibenz- [ b , f ] azepine-5-propanamine ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 19 H 24 N 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
|
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 280.40 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
174.5 ° C |
|||||||||||||||||||||
| pK s value |
9.4 |
|||||||||||||||||||||
| solubility |
Water: 18.2 mg l −1 (24 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data |
|
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Chemically, imipramine belongs to the class of dibenzazepines and is a drug from the group of tricyclic antidepressants .
history
Imipramine was the first modern drug for the treatment of depression and became the prototype for a whole class of psychiatric drugs . It was launched on the German market under the Tofranil brand . The developer and manufacturer was the Swiss company Geigy (now Novartis ); the market launch took place in 1958.
As an antidepressant , it was discovered by chance. The psychiatrist Roland Kuhn wanted to use it in 1957 as a neuroleptic in schizophrenic patients. During clinical testing it was found that it was unsuitable for this purpose, but worked well against depressive symptoms. Imipramine is structurally an analogue of promazine (bridged with –CH 2 –CH 2 - instead of –S–).
Extraction and presentation
A two-step synthesis starts from 10,11-dihydro-5 H -dibenz [ b , f ] azepine, which is first deprotonated with sodium amide and then reacted with 3-dimethylaminopropyl chloride.
Indications
Imipramine is approved for the treatment of all forms of depressive illnesses, for pavor nocturnus and enuresis nocturna , as well as for the adjuvant treatment of chronic pain .
An application for anxiety states and phobias is common, but happens without appropriate approval and thus off-label , as is the application for retrograde ejaculation .
According to a 2008 study, patients with panic disorder who take imipramine and take cognitive behavioral therapy (CBT) are more likely to relapse than those who only do CBT.
Imipramine is also used to treat cataplexy in narcolepsy .
Effects
In the CNS, imipramine inhibits the uptake of monoamines from the synaptic gap into the presynaptic vesicles and thus causes an increase in the concentration of the neurotransmitters serotonin and noradrenaline in the plasma. The relative deficiency of these messenger substances observed in depression is now compensated for by the increased availability. The improved neuronal transmission ultimately leads to an alleviation of depressive symptoms .
Imipramine intervenes in other transmission processes in the brain and acts z. B. anticholinergic (as an antagonist of certain acetylcholine effects) and antihistaminic . This creates the characteristic side effects of tricyclic antidepressants. Imipramine also acts as a FIASMA (functional inhibitor of acid sphingomyelinase ).
The activating and dampening partial effects are roughly balanced. Mood-enhancing substances with a similar drive-neutral effect are also referred to as imipramine-type antidepressants - sometimes even those that do not belong to the tricyclics.
The active sedative component usually diminishes over the course of the application; the mood-enhancing effect also only occurs after an intake of about 2–3 weeks.
Dosage forms
Imipramine exists as a generic and as an original preparation (including Tofranil ® ) in the form of tablets or dragees for oral use .
pregnancy and breast feeding period
There is clear evidence of risks to the human fetus, but the therapeutic benefits to the mother may outweigh the risks. Newborns whose mothers had taken imipramine until birth had symptoms such as breathing disorders, lethargy, colic, irritability, hypotension, hypertension, tremors, or convulsions in the first few hours or days. To avoid these symptoms, imipramine should be discontinued at least 7 weeks before the expected due date, if this is justifiable. Imipramine and its metabolite desmethylimipramine are excreted in human milk in small amounts. As nothing is known about the clinical relevance for the infant, breastfeeding or the drug should be discontinued.
Responsiveness
You may experience blurred vision, drowsiness, or other central nervous symptoms. In this case, patients should not drive a motor vehicle, operate machines or do any activities that require their full attention.
unwanted effects
Imipramine mainly has vegetative side effects:
- Feeling thirsty , dry mouth
- Hypotension , tachycardia , cardiac arrhythmias
- Mydriasis , accommodation disorders
- Constipation , but also diarrhea, etc. a. Gastrointestinal problems
- Micturition disorders up to urinary retention
- Loss of libido , erectile dysfunction (impotence)
The following can also occur:
- Dizziness, headache
- Restlessness, ataxia, tremor
- Weight gain
- skin rash
- Changes in the blood count , e.g. B. leukopenia or agranulocytosis
- Conduction disorders in the heart .
Psychological disturbances are tiredness, but also aggressive behavior and confusion (rarely: pharmacogenic delirium ). Imipramine can cause a change from a depressive phase to a manic phase in bipolar diseases .
criticism
The Federal Institute for Drugs and Medical Devices (BfArM) warns of suicidal behavior in children and adolescents. According to the Federal Institute, the Scientific Committee for Medicinal Products for Human Use (CHMP) has attested that the drug paroxetine contains an increased risk of side effects, such as suicide attempt, suicidal thoughts and hostility in children and adolescents, in a risk assessment process.
A team of researchers from the UK and the US concluded that the drug imipramine (and paroxetine) was neither effective nor safe for children and adolescents.
The above team came to the conclusion that the drug paroxetine (and imipramine) were no more effective than giving a dummy drug in the treatment of severe depression. At most, it achieves a placebo effect in the patient. On top of that, treatment with both drugs leads to severe side effects. For example, according to the research team, paroxetine leads to behavioral problems and a tendency to suicide, while imipramine triggers cardiac arrhythmias.
Genotoxic potential
In the experiment on Drosophila , imipramine caused genetic damage. Taking imipramine may increase the risk of breast cancer. According to the technical information for imipramine, however, there is no evidence that the active ingredient damages the genetic makeup or causes cancer.
Trade names
Tofranil (D, CH) and a generic (D)
Individual evidence
- ↑ a b c Entry on imipramine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Data sheet Imipramine hydrochloride from Sigma-Aldrich , accessed on April 5, 2011 ( PDF ).
- ↑ a b c d e f g h i A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications. 4th edition. Thieme-Verlag Stuttgart 2000, ISBN 1-58890-031-2 .
- ^ Gustav Ehrhart / Heinrich Ruschig : Medicines , 1968
- ↑ Hans-Jürgen Möller, Gerd Laux, Arno Deister, Hellmuth Braun-Scharm: Psychiatry and Psychotherapy. 4th edition. 2009, ISBN 978-3-13-128544-7 , p. 14.
- ↑ SD Raffa, JA Stoddard, KS White et al: Relapse following combined treatment discontinuation in a placebo-controlled trial for panic disorder. In: J Nerv Ment Dis. 196 (7), Jul 2008, pp. 548-555. PMID 18626295 .
- ↑ http://www.psychosoziale-gesundheit.net : Narcolepsy
- ↑ J. Kornhuber, M. Muehlbacher, S. Trapp, S. Pechmann, A. Friedl, M. Reichel, C. Mühle, L. Terfloth, T. Groemer, G. Spitzer, K. Liedl, E. Gulbins, P Tripal: Identification of novel functional inhibitors of acid sphingomyelinase . In: PLoS ONE . tape 6 , no. 8 , 2011, p. e23852 , doi : 10.1371 / journal.pone.0023852 .
- ↑ a b c Technical information from Tofranil on the drug information publication platform of the Swiss medical aid institute Swissmedic. Status: June 2012. Accessed March 22, 2014.
- ↑ bfarm.de
- ↑ study329.org
- ↑ CR Sharpe, JP Collet, E. Belzile, JA Hanley, JF Boivin: The effects of tricyclic antidepressants on breast cancer risk. In: British Journal of Cancer . Volume 86, Number 1, January 2002, pp. 92-97, doi: 10.1038 / sj.bjc.6600013 . PMID 11857018 , PMC 2746543 (free full text).
