Bergamot
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Bergamot | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 21 H 22 O 4 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 338.40 g mol −1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Bergamot is a chemical compound from the group of furocoumarins with a geraniol side chain. Bergamot occurs naturally in bergamot and grapefruit , and in smaller quantities in other citrus fruits .
properties
Bergamottine, like 6 ', 7'-dihydroxybergamottine , is an inhibitor of CYP3A4 and is partly responsible for the slower breakdown of some drugs after consuming grapefruit. Bergamottine increases the oral bioavailability and plasma concentration of these drugs. The IC 50 of bergamottine at CYP3A4 is less than 10 μM . The IC 50 for bergamottin in CYP1B1 is 13.86 μM.
Bergamottin, by extraction with ethyl acetate from the juice of grapefruit, followed by silica gel - chromatography and HPLC are isolated. Bergamottine is detected by HPLC with a UV detector or by mass spectrometry .
biosynthesis
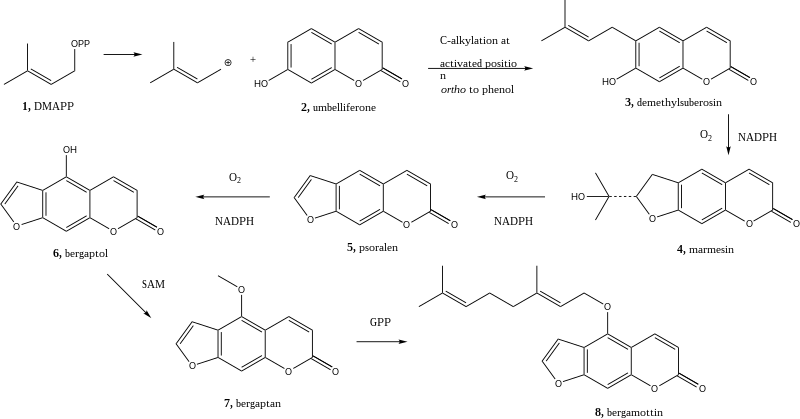
Bergamot is formed via the shikimate path . The Demethylsuberosin (3) is prepared by alkylation of umbelliferone formed (2). The alkylation begins with dimethylallyl pyrophosphate . Marmesin (4) generates a ring closure with the consumption of NADPH and oxygen by a cytochrome P450 monooxygenase . This reaction is then repeated twice, on the one hand to remove the hydroxyisopropyl group from marmesin (4), whereby psoralen (5) is formed, and on the other hand to add a hydroxyl group , whereby bergaptol (6) is formed. Bergaptol is then methylated with S-adenosylmethionine (SAM) , resulting in Bergapten (7). Bergamottine is formed through reaction with geranyl pyrophosphate (8).
Individual evidence
- ↑ a b Data sheet Bergamottin, analytical standard at Sigma-Aldrich , accessed on June 10, 2018 ( PDF ).
- ^ DG Bailey, J. Malcolm, O. Arnold, JD Spence: Grapefruit juice-drug interactions. In: British journal of clinical pharmacology. Volume 46, Number 2, August 1998, pp. 101-110, PMID 9723817 , PMC 1873672 (free full text).
- ↑ S. Zhou, S. Yung Chan, B. Cher Goh, E. Chan, W. Duan, M. Huang, HL McLeod: Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. In: Clinical Pharmacokinetics . Volume 44, Number 3, 2005, pp. 279-304, PMID 15762770 .
- ↑ MF Paine, WW Widmer, HL Hart, SN Pusek, KL Beavers, AB Criss, SS Brown, BF Thomas, PB Watkins: A furanocoumarin-free grapefruit juice establishes furanocoumarins as the mediators of the grapefruit juice-felodipine interaction. In: The American journal of clinical nutrition. Volume 83, Number 5, May 2006, pp. 1097-1105, doi : 10.1093 / ajcn / 83.5.1097 , PMID 16685052 .
- ↑ Y. Yamaguchi: Synthesis of Furanocoumarin, Benzofuran and Coumarin Derivatives Possessing an Inhibitory Effect on Human CYP, and Elucidation of the Inhibitory Mechanism. In: Yakugaku Zasshi. Volume 137, Number 10, 2017, pp. 1209-1214, doi : 10.1248 / yakushi.17-00135 , PMID 28966261 .
- ^ WL Hung, JH Suh, Y. Wang: Chemistry and health effects of furanocoumarins in grapefruit. In: Journal of food and drug analysis. Volume 25, number 1, January 2017, pp. 71-83, doi : 10.1016 / j.jfda.2016.11.008 , PMID 28911545 .
- ↑ P. Dewick: Medicinal Natural Products: A Biosynthetic Approach , 2nd Edition, Wiley & Sons, West Sussex, England, 2001. ISBN 978-0470741672 . P. 145.
- ↑ Emile Bisagni: Synthesis of psoralens and analogues. In: Journal of Photochemistry and Photobiology B: Biology. 14, 1992, pp. 23-46, doi : 10.1016 / 1011-1344 (92) 85081-5 .
- ^ AI Voznesensky, JB Schenkman: The cytochrome P450 2B4-NADPH cytochrome P450 reductase electron transfer complex is not formed by charge-pairing. In: Journal of Biological Chemistry . Volume 267, Number 21, July 1992, pp. 14669-14676, PMID 1321814 .
- ↑ UM Kent, HL Lin, KR Noon, DL Harris, PF Hollenberg: Metabolism of bergamottin by cytochromes P450 2B6 and 3A5. In: The Journal of pharmacology and experimental therapeutics. Volume 318, Number 3, September 2006, pp. 992-1005, doi : 10.1124 / jpet.105.099887 , PMID 16785317 .