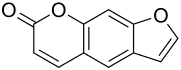
Psoralen
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Psoralen | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 11 H 6 O 3 | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 186.17 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
163-164 ° C |
||||||||||||||||||
| solubility |
soluble in ethanol , acetonitrile , good in water (1930 g l −1 ) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Psoralen is the main body of a group of natural substances found in the essential oils of various plants . The basic structure corresponds to coumarin with an added furan ring .
Occurrence
Psoralen is found in the Asian legume Psoralea corylifolia and in more than 20 plant species such as lime , angelica (angelica) and cloves .
use
Psoralens serve higher plants as defense substances against insect damage and fungal attack. In chemistry, it is used to study the structure and function of nucleic acids . Some studies with this active ingredient have shown better results in multiple sclerosis . Psoralen inhibits the transport of potassium and can make nerve fibers conductive again if they are not completely interrupted. The required dose is around 20 mg per day. Psoralens are used in processes for pathogen inactivation of platelet concentrates from apheresis or pooled buffy coats from whole blood donations.
hazards
Psoralen is the basic substance of the linear furanocoumarins , which also include bergapten and xanthotoxin . Like these, psoralen has photosensitizing properties, meaning that it sensitizes the skin to sunlight and UV radiation. The substance causes severe inflammation and sunburn when exposed to light.
Medical use
For the reasons mentioned above, psoralen can be used together with long-wave UV light ("Psoralen plus UVA", abbreviated PUVA ) as a form of photo-activated chemotherapy for the treatment of psoriasis , vitiligo , mastocytosis , lichen planus and other diseases.
Individual evidence
- ↑ a b Entry on psorals. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ Psoralen data sheet (PDF) from Carl Roth , accessed on December 14, 2010.
- ↑ a b Psoralen data sheet from Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ↑ Adam, Olaf: Dietary Guidelines in Multiple Sclerosis. German Medicine Verlag dmv, Münster 2007, page 28.
- ↑ Egon Fahr: Psoralens: Photobiological and dermatological effects. In: Pharmaceutical newspaper. Volume 127, No. 3, 1982, pp. 163-170.
literature
- Andreas Herde: Investigation of the coumarin pattern in fruits of selected Apiaceae. Dissertation University of Hamburg, 2005 (full text pdf 2.3 MB)
- Jeannette Aryee-Boi: Shower PUVA: An innovative method of local PUVA therapy Clinical and pharmacokinetic results in psoriasis vulgaris. Dissertation FU Berlin, 2002 (digital dissertation)
