Mukaiyama aldol reaction
| Educts |
|---|
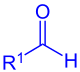 Aldehyde ( R 1 = alkyl, aryl) or formic acid ester ( R 1 = OR) |
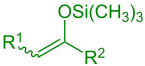 Silylated enol ( R 1 = alkyl, aryl, H; R 2 = alkyl, aryl, H, OR, SR) |
The Mukaiyama aldol reaction , also known as the Mukaiyama reaction , is a name reaction in organic chemistry and was discovered in the early 1970s by the Japanese chemist Teruaki Mukaiyama .
It is a form of the crossed aldol reaction that is one of the most important reactions for the formation of carbon-carbon bonds.
The Mukaiyama-Aldol reaction is related to the Mukaiyama-Michael reaction .
Overview
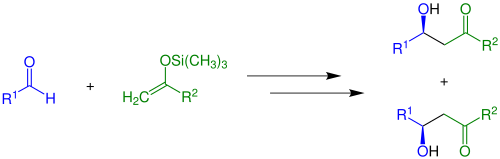
In the Mukaiyama aldol reaction, silylated enols are added to carbonyl compounds in the presence of a Lewis acid . The reaction allows a large number of possible residues (see starting materials). In the simplest case ( R 2 = H) and without the use of chiral catalysts , the reaction proceeds as follows:
During the reaction, a mixture of two reaction products, more precisely a racemate, is formed . If mixtures of cis and trans isomers of the respective ensiled enols are used, four reaction products are formed:
Both the syn - diastereomer and anti diastereomer occur as a racemate. Whether the anti diastereomer or the syn diastereomer is preferably formed in the reaction depends on the starting materials, reagents and the prevailing reaction conditions.
In the original work, stoichiometric amounts of Lewis acids such as TiCl 4 , SnCl 4 or the like were used. However, different catalytic versions were developed in later studies. It was possible to use both different Lewis acids and Lewis bases to carry out the reaction.
mechanism
One possible mechanism is formulated by László Kürti and Barbara Czakó in the book Strategic Applications of Named Reactions in Organic Synthesis . For the sake of clarity, only the simpler variant for the case R 2 = H is shown in the mechanism :
In the present example of the reaction mechanism, the Lewis acid TiCl 4 is used as the catalyst. In the first reaction step, the carbonyl group of the aldehyde is activated by the added Lewis acid. The double bond of the silane then attacks the activated carbonyl group and forms a new carbon-carbon bond. Reagent 1 is formed by splitting off a silyl chloride . Work-up, i.e. hydrolysis , gives a racemic mixture of aldols 2 and 3 .
application
The reaction is of great importance in modern synthetic chemistry. The stereochemistry can be influenced particularly effectively by using chiral Lewis acids as a catalyst. Using an enantiomer of a chiral catalyst, here marked as L * , the reaction proceeds according to the following reaction scheme:
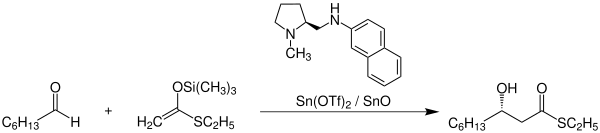
One of the many applications of the Mukaiyama aldol reaction is found for example in the synthesis of the natural product Sphingofungin B . The following reaction scheme shows the synthesis of an important intermediate in the sphingofungin B synthesis:
The Mukaiyama aldol reaction is used here mainly because of its excellent stereoselectivity . Using an ( S ) -proline derivative as a chiral ligand on the catalyst, the product shown can be synthesized with a yield of 87% and an enantiomeric excess of 94%. The abbreviation Tf used here stands for triflate .
Individual evidence
- ↑ a b Zerong Wang: Comprehensive organic name reactions and reagents . John Wiley & Sons, Hoboken, NJ 2009, ISBN 978-0-471-70450-8 , pp. 1991-1995 .
- ↑ a b Teruaki Mukaiyama, Koichi Narasaka, Kazuo Banno: New Aldol Type Reaction . In: Chemistry Letters . tape 2 , no. 9 , 1973, p. 1011-1014 , doi : 10.1246 / cl.1973.1011 .
- ↑ a b c d e László Kürti, Barbara Czakó: Strategic applications of named reactions in organic synthesis: background and detailed mechanisms . Elsevier Academic Press, Amsterdam / Boston 2005, ISBN 0-12-429785-4 , pp. 298-299 .
- ↑ a b Thomas Laue and Andreas Plagens: Name and catchword reactions in organic chemistry. Teuber Verlag, Wiesbaden 2006, ISBN 3-8351-0091-2 , p. 11.