Lomitapid
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
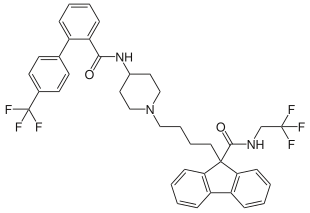
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Lomitapid | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 39 H 37 F 6 N 3 O 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| Drug class |
Other agents that affect lipid metabolism |
||||||||||||
| Mechanism of action |
MTP inhibitors |
||||||||||||
| properties | |||||||||||||
| Molar mass | 693.72 g · mol -1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Lomitapid is the first representative of the new class of active ingredients (“first-in-class”) of inhibitors of microsomal triglyceride transfer protein (MTP). MTP occurs in the lumen of the endoplasmic reticulum and is responsible for the binding and transport of individual lipid molecules between membranes.
Using a new approach, lomitapid, in combination with other measures, leads to an additional lowering of the LDL cholesterol level, which is greatly increased in the context of the rare homozygous familial hypercholesterolemia .
application areas
The US regulatory authority ( FDA ) approved lomitapid in the USA in December 2012 under the name Juxtapid , and the European Commission approved it as Lojuxta in July 2013 for the treatment of adult patients with homozygous familial hypercholesterolemia (HoFH); it must be given alongside a low-fat diet and other lipid-lowering drugs with or without low-density lipoprotein apheresis ( LDL apheresis ). Treatment with lomitapid should be started and supervised by a doctor experienced in the management of lipid disorders.
(→ for the clinical picture see homozygous familial hypercholesterolemia )
HoFH is a rare disease , which is why lomitapid was granted orphan drug status in the USA in 2011 . For Europe, however, this status was not granted.
The diagnosis of HoFH should, if possible, be genetically confirmed. Other forms of primary hyperlipoproteinemia as well as secondary causes of hypercholesterolaemia (e.g. nephrotic syndrome or hypothyroidism ) must be excluded.
In order to reduce the occurrence and severity of gastrointestinal side effects, the patient must consistently keep the fat content of the daily energy intake below 20 percent during use.
The safety and efficacy of lomitapide in children under 18 years of age have not been established; therefore this medicine is not recommended for use in children.
effectiveness
The single-arm, open-label registration study (study phase III) covered a period of 78 weeks of treatment. Six weeks before the start of treatment, the patients were instructed to continue their existing lipid-lowering therapy (apheresis used in 18 cases) without changes and to adhere to a low-fat diet (less than 20 percent of the daily supplied energy comes from fat). In addition, a daily supplementation of vitamin E and essential fatty acids was initiated. At the start of the study, the participants had an average LDL cholesterol level of 336 mg / dl. In patients who received the verum , the mean LDL cholesterol value fell significantly by 50 percent compared to the values measured at the beginning (from an average of 336 mg / dl to an average of 166 mg / dl; p <0.0001). In the so-called intention-to-treat (ITT) patient group (29 patients), the LDL cholesterol level fell by an average of 40 percent over 26 weeks (p <0.001). During the 78 weeks, 55 percent of ITT patients achieved LDL cholesterol values below 100 mg / dl, 31 percent below 70 mg / dl - and thus the target values specified in the European Dyslipidemia Guideline for patients with high and very high cardiovascular risk. 65 percent of the study patients were able to reduce their lipid-lowering accompanying therapy, some were able to stop the LDL apheresis or stretch the intervals.
There were considerable side effects such as gastrointestinal complaints in most patients up to an increase in liver enzymes to more than five times the upper normal value. The magnetic resonance imaging control showed that the hepatic lipid content increased from 1% before the study under treatment to over 8% after 72 weeks, since the active substance blocks the transport of cholesterol from the liver to the cells.
Mechanism of action
Lomitapid is a selective inhibitor of microsomal triglyceride transfer protein (MTP), an intracellular lipid transfer protein that occurs in the lumen of the endoplasmic reticulum and is responsible for the binding and transport of individual lipid molecules between membranes. The selective inhibition of MTP by Lomitapid leads to a reduced formation of lipid complexes - Very Low Density Lipoprotein (VLDL) in the liver, chylomicrons in the intestine. As a result, less VLDL is released from the liver into the blood, or fewer chylomicrons are absorbed from the intestine. This results in a consecutive lowering of the blood levels of VLD lipoprotein , low density lipoprotein (LDL), LDL cholesterol, chylomicrons and apolipoprotein B (Apo B).
Trade names
- Lojuxta (EU), Juxtapid ( CA , USA)
The marketing authorization holder is Aegerion Pharmaceuticals , a subsidiary of Novelion Therapeutics . For marketing in Europe was Lojuxta to Amryt Pharmaceuticals licensed.
literature
- FFSmith, J.McKenney, LA.Bloedon, WJSasiela, DJRader: Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. In: Nature Clinical Practice. 5 (8) 2008, pp. 497-505.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Summary of the European public assessment report (EPAR) for Lojuxta , der EMA, accessed on November 19, 2015.
- ↑ Summary of the EPAR for the public of the EMA (German), accessed on November 19, 2015.
- ^ FDA Grants Orphan Drug Designation to Aegerion Pharmaceuticals' Drug Candidate, Lomitapide, for Treatment of Familial Chylomicronemia , March 15, 2011.
- ↑ Doctors newspaper online: Waiver and Co .: Orphan drugs often without orphan status , from November 13, 2013, accessed on February 11, 2014.
- ↑ Marina Cuchel, Emma A Meagher u. a .: Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. In: The Lancet. 381, 2013, pp. 40-46, doi : 10.1016 / S0140-6736 (12) 61731-0 .
- ↑ Deutsches Ärzteblatt: New active ingredient lowers extreme cholesterol levels , November 2, 2012, accessed on February 2, 2014.
- ↑ M Mahmood Hussain, Paul Rava, Meghan Walsh, Muhammad Rana, Jahangir Iqbal: Multiple functions of microsomal triglyceride transfer protein. In: Nutrition & Metabolism. 9, 2012, p. 14, doi : 10.1186 / 1743-7075-9-14 .