Lotaustralin
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Lotaustralin | |||||||||
| other names |
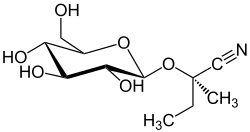
(2 R ) -2-methyl-2 - {[(2 S , 3 R , 4 S , 5 S , 6 R ) -3,4,5-trihydroxy-6- (hydroxymethyl) tetrahydro-2 H -pyran- 2-yl] oxy} butanenitrile |
|||||||||
| Molecular formula | C 11 H 19 NO 6 | |||||||||
| Brief description |
colorless needles |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 261.27 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
139 ° C |
|||||||||
| solubility |
readily soluble in ethyl acetate |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Lotaustralin is a cyanogenic glycoside , which is found in small quantities in the eponymous legume Lotus australis , in manioc ( Manihot esculenta ), in lima bean ( Phaseolus lunatus ), rose root ( Rhodiola rosea ), white clover ( Trifolium repens ) and other plants has been.
The epimer ( S ) -Epilotaustralin is mainly used passion flower and the two-grain Triticum dicoccum before.
Lotaustralin has the cyanohydrin of butanone as an aglycon , and β- D - glucose as the sugar unit and is therefore structurally related to linamarine . The only difference between the two glycosides is the aglycon, which is derived from propanone (linamarine) or butanone (Lotaustralin). Both homologs and the epimer epilotaustralin are also found in many plants, since the enzymes involved in the biosynthesis of the three substances are the same. These can convert isoleucine to Lotaustralin or Epilotaustralin, as well as Valine to Linamarine.
The enzyme linamarase can split both glycosides, producing the very poisonous hydrocyanic acid ; therefore Lotaustralin is one of the cyanogenic glycosides . The toxicity of Linamarin, Lotaustralin and Epilotaustralin are on a similar level.
Individual evidence
- ↑ a b c d Shmuel Yannai: Dictionary of Food Compounds with CD-ROM: Additives, Flavors, and Ingredients. CRC Press, 2003, ISBN 978-1-58488-416-3 , p. 688.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Frehner M, Scalet M, Conn EE: Pattern of the Cyanide-Potential in Developing Fruits: Implications for Plants Accumulating Cyanogenic Monoglucosides (Phaseolus lunatus) or Cyanogenic Diglucosides in Their Seeds (Linum usitatissimum, Prunus amygdalus) . In: Plant Physiol . 94, No. 1, 1990, pp. 28-34. PMID 16667698 .
- ↑ Akgul Y, Ferreira D, Abourashed E, Khan I: Lotaustralin from Rhodiola rosea roots . In: Fitoterapia . 75, No. 6, 2004, pp. 612-4. doi : 10.1016 / j.fitote.2004.06.002 . PMID 15351122 .
- ↑ Notes on poisoning: Trifolium repens . Canadian Poisonous Plants Information System. May 30, 2006. Archived from the original on February 7, 2012. Retrieved on February 11, 2007.
- ↑ a b J.B. Harborne, H. Baxter, GP Moss: Phytochemical dictionary: a handbook of bioactive compounds from plants. 2nd edition, CRC Press, 1999, ISBN 978-0-7484-0620-3 , p. 103.
- ↑ JPF D'Mello, CM Duffus, JH Duffus: Toxic substances in crop plants. Woodhead Publishing, 1991, ISBN 978-0-85186-863-9 , p. 208.
