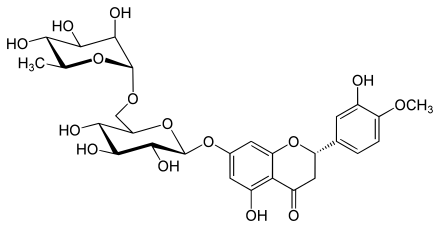
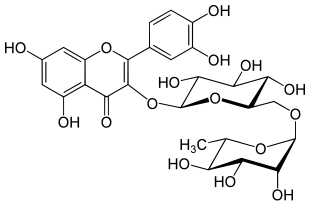
Rutinosis
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
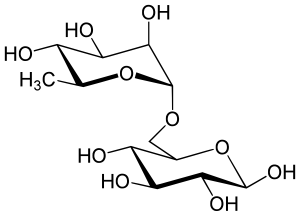
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Rutinosis | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 22 O 10 | |||||||||||||||
| Brief description |
beige solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 326.30 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.36 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
189–192 ° C (decomposition) |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Rutinose is a disaccharide and carbohydrate , which is composed of glucose and rhamnose with an α (1 → 6) bond. Rutinose produced during the hydrolysis of rutin with the enzyme Rhamnodiastase and was first reported by M. Schubert 2002. datisca isolated.
Occurrence
Rutinose is part of the glycoside hesperidin , which is found in the peel of oranges and lemons .
With quercetin , rutinose forms the glycoside rutin , which is found in many plants to protect against UV radiation .
literature
- Kurt Peter C. Vollhardt , Neil E. Schore : Organic Chemistry . ISBN 3-527-32754-1 , p. 1110 (English; limited preview in Google book search).
- Shintaro Kamiya, Sachiko Esaki, Reiko Tanaka: Synthesis of Some Disaccharides Containing an L-Rhamnopyranosyl or L-Mannopyranosyl Residue, and the Substrate-specificity of α-L-Rhamnosidase from Aspergillus niger . In: Agricultural and Biological Chemistry . tape 49 , no. 1 , 1985, pp. 55-62 , doi : 10.1271 / bbb1961.49.55 (English).
Web links
Commons : Rutinose - collection of images, videos and audio files
- Rutinose on Spektrum.de
Individual evidence
- ↑ a b c Datasheet Rutinose at Sigma-Aldrich , accessed on August 8, 2017 ( PDF ).
- ^ The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals: Physical Properties for More Than 54,000 Organic and Inorganic Chemical Compounds, Coverage for C1 to C100 Organics and Ac to Zr Inorganics . Gulf Professional Publishing, 2015, ISBN 978-0-12-801146-1 , pp. 386 ( Google Books ).
- ↑ Constitution and occurrence of organic plant substances: exclusive alkaloids . Springer-Verlag, 2013, ISBN 978-3-0348-6808-2 , pp. 261 ( Google Books ).
- ↑ Entry on rutinosis at TCI Europe, accessed on August 8, 2017.