Lysosomal α-glucosidase
| ATM | ||
|---|---|---|
| other names |
Glucosidase, alpha; Acid; Aglucosidase alfa; Acid maltase; EC 3.2.1.20; Glycogen Storage Disease Type II; Lysosomal alpha-glucosidase; Pompe Disease; LYAG |
|
| Properties of human protein | ||
| Mass / length primary structure | 952 amino acids, 105,324 Da | |
| Identifier | ||
| External IDs | ||
| Enzyme classification | ||
| EC, category | 3.2.1.20 , glycosidase | |
| Response type | hydrolysis | |
| Substrate | terminal 1,4-linked α-D-glucose residues | |
| Products | α-D-glucose | |
| Occurrence | ||
| Homology family | acid maltase | |
| Parent taxon | Euteleostomi | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 2548 | 14387 |
| Ensemble | ENSG00000171298 | ENSMUSG00000025579 |
| UniProt | P10253 | P70699 |
| Refseq (mRNA) | NM_000152 | NM_001159324 |
| Refseq (protein) | NP_000143 | NP_001152796 |
| Gene locus | Chr 17: 80.1 - 80.12 Mb | Chr 11: 119.27 - 11.93 Mb |
| PubMed search | 2548 |
14387
|
The lysosomal α-glucosidase (also acid maltase , gene : GAA ) is that enzyme , which in lysosomes long chain polysaccharides to glucose degrades. It is not part of the breakdown of glycogen in the liver, nor is it a part of the digestion of polysaccharides in the intestine (like maltase-glucoamylase ), but it helps break down foreign substances in the lysosomes. Acid maltase is found in vertebrates. In humans it is localized in all types of tissue. Mutations in the GAA gene can lead to type II glycogen storage disease ( Pompe disease ).
A potent inhibitor of acid maltase is the naturally occurring salacinol .
Catalyzed reaction
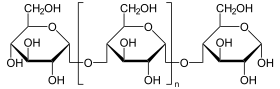 (n = m) + H 2 O (n = m-1) + glucose
(n = m) + H 2 O (n = m-1) + glucose

Terminal glucose is split off from polysaccharides.
Use as a medicine
The active ingredient is used as an orphan drug for the treatment of Pompe disease . The Federal Supreme Court (Switzerland) ruled in 2010 that the drug is not subject to health insurance for reasons of cost, because it was not on the list of specialties at the time.
Web links
Individual evidence
- ↑ UniProt P10253
- ↑ Minami Y, Kuriyama C, Ikeda K, et al : Effect of five-membered sugar mimics on mammalian glycogen-degrading enzymes and various glucosidases . In: Bioorganic & Medicinal Chemistry . 16, No. 6, March 2008, pp. 2734-40. doi : 10.1016 / j.bmc.2008.01.032 . PMID 18258441 .
- ^ Tomas Poledna, Marianne Tschopp: The Myozyme decision of the Federal Court. In: Jusletter February 7, 2011.
- ↑ in the extract also published as BGE 136 V 395
