Natural product nomenclature
The natural substance nomenclature describes the chemical nomenclature of natural substances . It does not follow the guidelines of the systematic chemical nomenclature, because the very complex structures would lead to very long and impractical names. Certain natural substances get their names from the authors who clarified their structure and described it for the first time in the scientific literature. A system is used for these substances that is based on trivial names for skeleton, to which the substituents are added. Substituents are to be understood as meaning both functional groups such as hydroxyl or oxo groups and esters of smaller acids and sugars. This semi-systematic nomenclature is also accepted by the IUPAC . There are flowing transitions between the semi-systematic nomenclature and trivial names, so that different more or less systematic names can be used for many natural substances. The common names of the aglyca are often used as part of the name, e.g. B. with flavones or terpene glycosides. The scaffolds are not necessarily named according to the methodology described under nomenclature. The numbering is based on biosynthesis or on traditions (e.g. the numbering of many diterpenes and triterpenes is based on the steroid numbering).
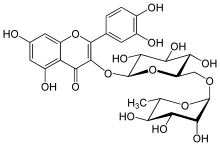
Example rutin :
- Systematic name according to IUPAC: 2- (3,4-dihydroxyphenyl) -5,7-dihydroxy-3 - [(2 S , 3 R , 4 S , 5 S , 6 R ) -3,4,5-trihydroxy-6 - [{(2 R , 3 R , 4 R , 5 R , 6 S ) -3,4,5-trihydroxy-6-methyloxan-2-yl} oxymethyl] oxan-2-yl] oxychromen-4-one
- Semi-systematic name: 3,3 ′, 4 ′, 5,7-Pentahydroxyflavone 3- O - [α- L -rhamnopyranosyl- (1 → 6) -β- D -glucopyranoside]
- Derived from the aglycon quercetin : Quercetin-3- O - [α- L -rhamnopyranosyl- (1 → 6) -β- D -glucopyranoside]
- Composed of the common name of the aglycone and the common name of the disaccharide: Quercetin-3-rutinosid
Framework
In the narrower sense, the basic structure in natural product chemistry is the carbon structure of a natural product. In many natural product classes, some heterocyclic rings are also included in the basic structure. In the case of alkaloids, e.g. B. all rings in which nitrogen is integrated also form the basic structure, in the case of flavones the oxygen in the pyrone ring. Not part of the basic structure, but as substituents (colloquially usually referred to as "residues") are, however, counted z. B. methylenedioxy rings on flavones or lignans or prenyl radicals cyclized to the chroman ring. Many aromatic systems are part of the basic structure if they are typical of the substance class. For example, ergot alkaloids (ergolines) contain an indole ring that is part of the basic structure. A typical ending for alkaloid structures is "-in", whereby not every natural product that ends with "-in" is an alkaloid or alkaloid structure (e.g. quercetin or coumarin ). In the case of terpenes, only the saturated hydrocarbon structure is considered to be the basic structure, even if there are representatives with aromatic rings. So z. B. the carnosic acid to the diterpene skeleton of the abietans. The names of the terpene skeletons therefore end in "-an" analogous to the hydrocarbons. Even with the ending "-an" there are natural substances or natural substance skeletons that are neither terpene nor saturated hydrocarbons, e.g. B. Lignan.
Prefixes for modified basic structures
If the basic structure was created by a subsequent reaction from the basic structure of another natural product, the structural difference is sometimes described by prefixes. The numbering of the original system is retained.
Nor
The prefix nor is used to describe the absence of a carbon atom. Most of the time, it is a question of missing methyl groups that have been split off by oxidative degradation ( decarboxylation ). For example, the basic structure derived from tropane , which does not have an N-methyl group on nitrogen, but only a proton, is called nor-tropane. If more than one carbon atom is missing compared to the basic structure, the usual numerical prefixes from chemical nomenclature are used. Limonoids are derived from tetracyclic triterpenes , which are missing 4 C atoms from the chain attached to the five-membered ring (D-ring), which is why they are also known as tetra-nor triterpenes.
homo
Conversely to nor , homo is used, i.e. for carbon atoms added to the basic structure. However, homo is only used if the additional carbon atom has been attached to the backbone via a CC link, i.e. H. N - or O - methylations count as substituents and are not referred to as homo . Examples of homo-cholestans are ergosterol or sitosterol , which have one or two additional carbon atoms in the form of a methyl or ethyl group compared to cholesterol .
Seco
When a CC bond is split, the prefix seco (from seco, Latin for cutting) is used. The best-known example are the seco-iridoids, which are derived from the iridoids by cleaving the bond between C-6 and C-7 (cf. Loganin and Seco-Loganin). Since the numbering of the original framework is retained, it can sometimes not be understood as systematic at first glance.
Cyclo
Additionally closed rings, which are formed by linking two carbon atoms, can be described by the prefix cyclo , e.g. B. in the 2,2'- or 2,7'-cyclolignans, which are derived from the lignans . Usually the new scaffolding is given a new name.
Abeo
Abeo is used when a CC binding has been "moved". An example are the 11 (15 → 1) -Abeo-taxanes derived from the taxane . In the taxane there is a CC bond between C-11 and C-15, in the 11 (15 → 1) -Abeo-taxane there is a bond between the carbon atoms C-11 and C-1 instead.
Epi
The prefix epi describes natural substances that differ from the corresponding basic structure at a stereocenter. Usually this prefix does not refer to the nomenclature in basic structures, but in functionalized natural substances. For example, the epicatechin is the epimer of the catechin. Since there are two stereocenters in catechin, the correct designation is 3-epi-catechin.
numbering
The numbering of the basic structures is based either on the (supposed) biosynthesis, or based on traditional numbering for natural substances that have been known for a long time, or begins at a position that is often more or less arbitrarily determined by the discoverer of the structure. The numbering of most natural product frameworks can be found in the freely available documentation on the Dictionary of Natural Products (see web links).
Examples of biosynthesis-based numbering
Lignans are formed by the dimerization of two phenylpropanes. The numbering refers to these two components and numbers them from 1 to 9 or from 1 'to 9'.
Some diterpene frameworks are formed by cyclization of the 14-membered membrane ring. The membrane ring is numbered according to the systematic IUPAC nomenclature. This system is z. B. in the taxane system, while other diterpene structures, which are also derived from the Cembran, z. B. Tigliane and Fusicoccoane , are counted differently.
Examples of numbering based on traditional steroid numbering
Many sesqui, di- and triterpene structures are numbered in analogy to steroids, ie at the top of the A-ring they start with 1, then count through the molecule, starting counterclockwise. In the case of triterpenes and diterpene structures, which also have a decalin system, the analogy to the steroid is still easy to see. In some other scaffolding, e.g. B. the 10-ring sesquiterpene skeleton Germacran, it is only understandable if the ring is drawn in the correct orientation.
Glycosides
Many natural products have linear or branched sugar chains as substituents. IUPAC also has recommendations for semi-systematic nomenclature. Trivial names are used for the monosaccharides that are often found in natural substances. It is also common to use three-letter abbreviations for the monosaccharides. The absolute stereochemistry is described by D or L, the linkage at the anomeric center by the α / β nomenclature . In addition, a small italic f or p indicates whether the sugar is pyranose (6-ring) or furanose (five-ring).
The linking of the sugars in linear or branched chains is described as follows:
- If the sugars are after the designation of the basic structure, they get the ending "side", if they are in front of them, the ending "osyl" is used
- The sugar that is directly linked to the basic structure is at the end of the sugar chain name
- Branches are separated from each other by nested brackets, if necessary
Example rutin (aglycon quercetin with two sugars): Quercetin 3- O - [α- L -rhamnopyranosyl- (1-6) -β- D -glucoside]
Example rebaudioside A (aglycon steviol with a branched glycoside of 3 glucoses at C-13 of the kauran and a single glucose as an ester at C-18 of the kauran structure): 19- O -β- D -Glucopyranosyl-13- O - (β- D -glucopyranosyl (1-2) -β- D -glucopyranosyl (1-3)) -β- D -glucopyranosyl-13-hydroxykaur-16-en-19-acid
Web links
- The most comprehensive overview of the known natural product frameworks can be found on the Dictionary of Natural Products page: http://dnp.chemnetbase.com/HelpFiles/DNP_Introduction.pdf , (accessed on January 16, 2018).
Individual evidence
- ^ HA Favre, PM Giles, K.-H. Hellwich, AD McNaught, GP Moss: Revised Section F: Natural products and related compounds (IUPAC Recommendations 1999). Corrections and modifications (2004) . In: Pure and Applied Chemistry . tape 76 , no. 6 , January 1, 2004, doi : 10.1351 / pac200476061283 .
- ^ AD McNaught: Nomenclature of carbohydrates (IUPAC Recommendations 1996) . In: Pure and Applied Chemistry . tape 68 , no. 10 , January 1, 1996, doi : 10.1351 / pac199668101919 .