Flavones
Flavones are yellow plant dyes , as derivatives of flavone to the class of flavonoids include. About 300 naturally occurring flavones are known. Like other flavonoids, most flavones occur as water-soluble glycosides , e.g. B. hyperoside and quercitrin , hesperidin , luteolin , chrysin . They often appear as copigments of anthocyanins . The interaction of both types of dye explains the simultaneous appearance of yellow and red in different flowers.
The flavones are also to be understood as derivatives of the chromone .
Examples
| Surname | structure | R 5 | R 6 | R 7 | R 3 ' , R 5' | R 4 ' |
|---|---|---|---|---|---|---|
| Apigenin | 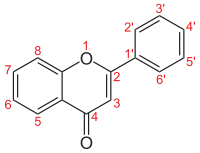 |
-OH | - | -OH | -H, -H | -OH |
| Acacetin | -OH | - | -OH | -H, -H | -OCH 3 | |
| Genkwanin | -OH | - | -OCH 3 | -H, -H | -OH | |
| Luteolin | -OH | - | -OH | -H, -OH | -OH | |
| Chrysoeriol | -OH | - | -OH | -H, -OCH 3 | -OH | |
| Diosmetin | -OH | - | -OH | -H, -OH | -OCH 3 | |
| Tricetin | -OH | - | -OH | -OH OH | -OH | |
| Tricine | -OH | - | -OH | -OCH 3 , -OCH 3 | -OH | |
| Scutellar pure | -OH | -OH | -OH | -H, -H | -OH | |
| Eupatorin | -OH | -OCH 3 | -OCH 3 | -H, -OH | -OCH 3 | |
| Sinensetin | -OCH 3 | -OCH 3 | -OCH 3 | -H, -OCH 3 | -OCH 3 | |
| Chrysin | - | -OH | - | -OH | -H, -H | - |
| Tectochrysin | - | -OH | - | -OCH 3 | -H, -H | - |
See also
Individual evidence
- ↑ Hermann Ammon (Ed.): Hunnius Pharmaceutical Dictionary . 8th edition, de Gruyter, Berlin 2004. ISBN 3-11-015792-6 .
- ↑ Entry on Flavones. In: Römpp Online . Georg Thieme Verlag, accessed December 10, 2014.
- ^ Matthias Melzig, Eberhard Teuscher and Ulrike Lindequist: Biogenic medicines: A textbook of pharmaceutical biology . Scientific Publishing Company; 6. completely rework. Edition 2004; ISBN 3-8047-2073-0 ; S 305.
