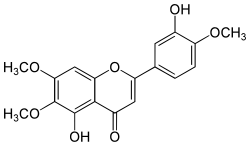
Eupatorin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Eupatorin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 18 H 16 O 7 | ||||||||||||||||||
| Brief description |
dark yellow solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 344.32 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Eupatorin is a chemical compound from the group of flavones . Eupatorin comes in various plant species of the tribe Eupatorieae before, including the genus Eupatorium ( Eupatorium semiserratum , Eupatorium altissimum ), but also in Merrillia caloxylon , Hyptis tomentosa , Lantana montevidensis , Centaurea arenaria , Lippia dulcis , polium Teucrium , Salvia limbata , Tanacetum vulgare and Orthosiphon stamineus .
Eupatorin affects mitosis and leads to polyploidy and apoptosis . Eupatorin has an antiproliferative effect against tumor cells in mammalian cells and is broken down by cytochrome P1 . At the same time it inhibits cytochrome P1 and STAT1 α. Eupatorin inhibits the gene expression of iNOS and COX-2 and thus has an anti-inflammatory effect .
Individual evidence
- ↑ a b c d Eupatorin data sheet , analytical standard at Sigma-Aldrich , accessed on March 8, 2018 ( PDF ).
- ↑ SM Kupchan, JR Knox, MS Udayamurthy: Tumor inhibitors. 8. Eupatorin, new cytotoxic flavones from Eupatorium semiserratum. In: Journal of pharmaceutical sciences. Volume 54, Number 6, June 1965, pp. 929-930, PMID 5847037 .
- ^ JH Adams, JR Lewis: Eupatorin, a constituent of Merrillia caloxylon. In: Planta medica. Volume 32, Number 1, August 1977, pp. 86-87, doi : 10.1055 / s-0028-1097564 , PMID 905420 .
- ^ DG Kingston, MM Rao, WV Zucker: Plant anticancer agents. IX. Constituents of Hyptis tomentosa. In: Journal of natural products. Volume 42, Number 5, 1979 Sep-Oct, pp. 496-499, PMID 521819 .
- ↑ T. Nagao, F. Abe, J. Kinjo, H. Okabe: Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis Briq. and consideration of structure-activity relationship. In: Biological & pharmaceutical bulletin. Volume 25, Number 7, July 2002, pp. 875-879, PMID 12132661 .
- ↑ B. Csapi, Z. Hajdú, I. Zupkó, A. Berényi, P. Forgo, P. Szabó, J. Hohmann: Bioactivity-guided isolation of antiproliferative compounds from Centaurea arenaria. In: Phytotherapy research: PTR. Volume 24, Number 11, November 2010, pp. 1664-1669, doi : 10.1002 / ptr.3187 , PMID 21031625 .
- ↑ M. Ono, H. Morinaga, C. Masuoka, T. Ikeda, M. Okawa, J. Kinjo, T. Nohara: New Bisabolane-Type Sesquiterpenes from the Aerial Parts of Lippia dulcis. In: Chemical & pharmaceutical bulletin. Volume 53, Number 9, September 2005, pp. 1175-1177, PMID 16141591 .
- ↑ E. Verykokidou-Vitsaropoulou, C. Vajias: Methylated flavones from Teucrium polium. In: Planta medica. Number 5, October 1986, pp. 401-402, doi : 10.1055 / s-2007-969198 , PMID 17345353 .
- ↑ AR Gohari, p Saeidnia, M. Malmir, A. Hadjiakhoondi, Y. Ajani: flavones and rosmarinic acid from Salvia limbata. In: Natural Product Research . Volume 24, Number 20, December 2010, pp. 1902-1906, doi : 10.1080 / 14786411003766912 , PMID 21108116 .
- ↑ GR Schinella, RM Giner, MC Recio, P. Mordujovich de Buschiazzo, JL Ríos, S. Máñez: Anti-inflammatory effects of South American Tanacetum vulgare. In: The Journal of pharmacy and pharmacology. Volume 50, Number 9, September 1998, pp. 1069-1074, PMID 9811170 .
- ↑ Y. Tezuka, P. Stampoulis, AH Banskota, S. Awale, KQ Tran, I. Saiki, S. Kadota: Constituents of the Vietnamese medicinal plant Orthosiphon stamineus. In: Chemical & pharmaceutical bulletin. Volume 48, Number 11, November 2000, pp. 1711-1719, PMID 11086900 .
- ↑ AL Salmela, J. Pouwels, A. Kukkonen-Macchi, S. Waris, P. Toivonen, K. Jaakkola, J. Mäki-Jouppila, L. Kallio, MJ Kallio: The flavonoid eupatorin inactivates the mitotic checkpoint leading to polyploidy and apoptosis. In: Experimental cell research. Volume 318, Number 5, March 2012, pp. 578-592, doi : 10.1016 / j.yexcr.2011.12.014 , PMID 22227008 .
- ↑ V. Androutsopoulos, RR Arroo, JF Hall, S. Surichan, GA Potter: Anti-proliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism. In: Breast cancer research: BCR. Volume 10, number 3, 2008, p. R39, doi : 10.1186 / bcr2090 , PMID 18454852 , PMC 2481486 (free full text).
- ↑ VP Androutsopoulos, A. Papakyriakou, D. Vourloumis, DA Spandidos: Comparative CYP1A1 and CYP1B1 substrate and inhibitor profile of dietary flavonoids. In: Bioorganic & medicinal chemistry. Volume 19, Number 9, May 2011, pp. 2842-2849, doi : 10.1016 / j.bmc.2011.03.042 , PMID 21482471 .
- ↑ a b M. Laavola, R. Nieminen, MF Yam, A. Sadikun, MZ Asmawi, R. Basir, J. Welling, H. Vapaatalo, R. Korhonen, E. Moilanen: Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation. In: Planta medica. Volume 78, Number 8, May 2012, pp. 779-786, doi : 10.1055 / s-0031-1298458 , PMID 22516932 .
