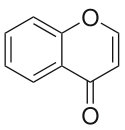
Chromone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Chromone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 6 O 2 | ||||||||||||||||||
| Brief description |
white to yellow powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 146.14 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.29 g cm −3 |
||||||||||||||||||
| Melting point |
55-60 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Chromone is a chemical compound belonging to the benzopyron group in which a benzene ring ( aromatic ) is theoretically condensed with 4-pyrone (an oxygen-containing heterocycle ) (hence also benzo-γ-pyrone ).
It is a derivative of 4 H -chromene (4-benzopyran), which has a keto group in position 4 instead of two hydrogen atoms .
Isomerism
Chromone is constitutional isomer with coumarin .
Derivatives
Derivatives of chromone (group of substances: chromones ) are usually made from phenols and β-keto acid esters by heating with phosphorus pentoxide .
The derivatives occurring in nature, for example in diamonds, include the flavones and isoflavones (yellow plant pigments ) as well as khellinin ( glycoside active in the heart ) and the related, highly toxic kellin .
Artificially produced chromones are the antiallergic drugs cromoglicic acid and nedocromil (in pharmacology : cromone ).
Individual evidence
- ↑ SCBT: Chromones
- ^ Carl L. Yaws: Thermophysical properties of chemicals and hydrocarbons. Andrew, Norwich NY 2008, ISBN 978-0-8155-1596-8 , p. 242.
- ↑ a b c d Datasheet Chromon at Sigma-Aldrich , accessed on September 19, 2011 ( PDF ).
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 1: A-Cl. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1979, ISBN 3-440-04511-0 , p. 744.
- ↑ Alexander I. Gray: Structural diversity and distribution of coumarines and chromones in the Rutales. In: Peter G. Waterman, Michael F. Grundon (Eds.): Chemistry and chemical taxonomy of the Rutales. London / New York 1983 (= Annu. Proc. Phytochem. Soc. Eur. Volume 22), pp. 97-146.
- ↑ Lung Information Service : Cromone ( Memento of the original from December 22, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed December 15, 2015.
literature
- H. Beyer, W. Walter: Textbook of organic chemistry. 20th edition. Hirzel, Stuttgart 1984, pp. 743-744.
- R. Ebermann, I. Elmadfa: Textbook Food Chemistry and Nutrition 2nd Edition, Springer Vienna New York, 2001, ISBN 978-3-7091-0210-7 , pp. 206-214.