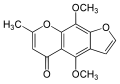
Khellinin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Khellosid ( Glycoside of Khellol) | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Khellinin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 19 H 20 O 10 | ||||||||||||||||||
| Brief description |
yellowish crystal powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 444.38 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
179 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
The khellinin or khellosid is a cardiac glycoside of chromone derivatives of various plants such as the bishop's herb ( Ammi visnaga ) and the winterling ( Eranthis hyemalis ). The aglycon of the khellinin is the khellol . The white to yellowish, odorless khellinin is soluble in acetic acid and hot ethanol , but little in water. The related khellin also found in bishop's herb is highly toxic.
In primates (such as macaques Cynomolgus and Macaca fascicularis ) khellinin was able to lower the plasma concentration of LDL and HDL lipoproteins .
literature
- K. Samaan, AM Hossein: The existence in Ammi visnaga of a cardiac depressant principle visammin and a cardiac stimulant glycoside khellinin. In: J Egypt Med Assoc. 33 (12) 1950, pp. 953-960. PMID 14814677
- A. Sibille et al.: Clinical trial of a new coronary vasodilator: khellinin. In: Brux Med. 34 (9) 1954, pp. 367-71. PMID 13140893
- Egon Stahl , Werner Schild (1986): Isolation and characterization of natural substances . Gustav Fischer Verlag, ISBN 3-437-30511-5 , p. 114.
Individual evidence
- ↑ a b Max Daunderer: Clinical Toxicology. Poison information, poison detection, poisoning therapy . ecomed, 1988, ISBN 3-609-70000-9 .
- ↑ a b c F. v. Bruchhausen, M. Albinus, H. Hager: Hager's handbook of pharmaceutical practice. Volume 4: Substances AK. 5th edition. Springer, 1999, ISBN 3-540-62644-1 , p. 762.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Entry on khellinin in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Journal of Drug Research. Vol. 7 (2), p. 1, 1975.
- ↑ External identifiers or database links for Khellol : CAS number: 478-79-5, PubChem : 164613 , ChemSpider : 144309 , Wikidata : Q83070196 .
- ↑ External identifiers of or database links to Khellin : CAS number: 82-02-0, EC number: 201-392-8, ECHA InfoCard: 100.001.267 , PubChem : 3828 , ChemSpider : 3696 , Wikidata : Q2079998 .
- ↑ Data sheet Khellin (PDF) from Carl Roth , accessed on March 17, 2010.