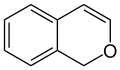
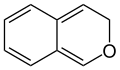
Chromene
| Chromene | |||
| Surname | 2 H- chromene | 4 H- chromene | |
| other names | 2-benzopyran | 4-benzopyran | |
| Structural formula |

|

|
|
| CAS number | 254-04-6 | 254-03-5 | |
| ? (Mixture of isomers) | |||
| PubChem | 9211 | 136068 | |
| Molecular formula | C 9 H 8 O | ||
| Molar mass | 132.16 g mol −1 | ||
| Physical state | liquid | ||
| Brief description | |||
| Melting point | <25 ° C | ? ° C | |
| boiling point | 92 ° C (13 mbar) | 77 ° C (10 mbar) | |
| density | 1.10 g cm −3 | ? g cm −3 | |
| solubility | insoluble in water | ||
|
GHS labeling |
|
||
| H and P phrases | see above | ||
| see above | |||
| see above | |||
| Toxicological data | 250 mg kg −1 ( LD 50 , mouse , ip ) | ||
The two organic chemical compounds 2 H chromene and 4 H chromene are referred to as chromenes (also: benzopyrans ) . These belong to the aromatic compounds and the heterocycles , since the ring system contains the heteroatom oxygen . Both substances are isomers that differ only in the position of the double bond in the heterocyclic six-membered ring.
Occurrence, derivatives and uses
2 H- Chromene occurs in the myrtle family Calyptranthes tricona . Derivatives of 2 H chromium act as potent potassium channel openers and are used as medicinal substances ( Cromakalim , Lemakalim ); Carbocromen is used for heart therapy. From 2 H -chromen guided coumarin and umbelliferone from from 4 H -chromen the chromone , the anthocyanidins and flavones . By hydrogenation of the pyran ring one obtains the chroman , which z. B. represents the basic building block of the tocopherols , the Rotenoids and some cannabinoids . Many alkaloids also contain a chromene structure as a chromophore .
Isochromes
Positional isomers of the two chromenes are the 1 H -isochromes and the 3 H -isochromes, in which the oxygen atom is in position 2.
presentation
A simple one-step synthesis of 2 H- chromene according to Yoshiyuki Kawase is possible by direct cyclization of salicylaldehyde with propene carboxylic acid esters in DMF . The synthesis of 2 H chromene is also carried out by a Wittig reaction and subsequent ring closure starting from the sodium salt of salicylaldehyde and vinyl triphenylphosphonium bromide.
literature
- Entry on Chromene. In: Römpp Online . Georg Thieme Verlag, accessed on January 2, 2015.
Individual evidence
- ↑ a b Entry for 1,2-benzopyran in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 31, 2018 or earlier.
- ↑ a b Scheweizer, EE: Reactions of Phosphorus Compounds. III. A New General Ring Synthesis from Vinyltriphenylphosphonium Bromide in J. Am. Chem. Soc. 86 (1964) 2744. doi : 10.1021 / ja01067a061
- ↑ DR Lide, GWA Milne: Handbook of Data on Organic Compounds. 3rd edition, CRC Press, 1994, ISBN 9780849304453 , p. 1548.
- ^ A b D. R. Lide, GWA Milne: Handbook of Data on Common Organic Compounds. CRC Press, 1995, ISBN 9780849304040 , p. 568.
- ↑ With regard to its dangerousness, the substance has not yet been classified by the EU, a reliable and citable source has not yet been found.
- ↑ European Journal of Medicinal Chemistry . Vol. 11, p. 81, 1976.
- ↑ a b Sabine Plücker: Investigations on the representation of radioligands from the series of potassium channel openers of the benzopyran type (PDF; 2.0 MB) , dissertation at Heinrich Heine University Düsseldorf , 2004.
- ^ IW Southon, GA Cordell, J. Buckingham: Dictionary of alkaloids. CRC Press, 1989, ISBN 9780412249105 .
- ↑ Kawase, Y., Yamaguchi, S., Horita, H., Takeno, J., Kameyama, H .: Bull. Chem. Soc. Jpn. , 1982, 55, 1153-1155.