Sinigrin
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
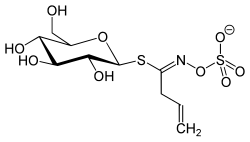
|
|||||||
| Structural formula without representation of the cation | |||||||
| General | |||||||
| Surname | Sinigrin | ||||||
| other names |
|
||||||
| Molecular formula | C 10 H 17 NO 9 S 2 (sulfonic acid) | ||||||
| Brief description |
colorless solid (potassium salt) |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 397.47 g · mol -1 ( potassium salt ) | ||||||
| Physical state |
firmly |
||||||
| Melting point |
125–127 ° C (potassium salt) |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Sinigrin is a mustard oil glycoside that is found in black mustard and horseradish , among other things .
In many plants of the cruciferous family it is one of the original substances for the pungent taste, which is felt when cell membranes are destroyed by eating or eating and can be understood as a self-protection of the plant.
Black mustard ( Brassica nigra ) contains a particularly large amount of sinigrin, and some oriental varieties of brown mustard ( Brassica juncea ) also have high levels.
From sinigrin is by the action of the enzyme myrosinase , which is stored in the cell of the plant in another place, with the elimination of glucose , the allyl isothiocyanate is released, the carrier of the sharp taste. This substance is also used therapeutically as allyl mustard oil , but is not stable in the long term and is further broken down in an aqueous medium, e.g. a. to allylamine , which is irritating to skin and eyes.
Allyl isothiocyanate released in the mouth also creates a sensory impression of sharpness in the throat and nose , unlike that from sinalbin , contained in white mustard ( Sinapis alba ), 4-hydroxybenzyl isothiocyanate, which has a significantly lower vapor pressure.
Individual evidence
- ↑ a b entry on glucosinolates. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren, Peter Wothers: Organic Chemistry. Oxford University Press, 2001, ISBN 0-19-850346-6 , pp. 1367-1368.

