Hydroxyproline
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| Structure with unspecified stereochemistry | |||||||||||||
| General | |||||||||||||
| Surname | Hydroxyproline | ||||||||||||
| other names |
4-hydroxypyrrolidine-2-carboxylic acid |
||||||||||||
| Molecular formula | C 5 H 9 NO 3 | ||||||||||||
| Brief description |
colorless solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 131.13 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
|
||||||||||||
| solubility |
soluble in water |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Hydroxyproline is a chiral α- amino acid that is chemically bound in collagen .
The exact name of the naturally occurring amino acid is L -4-hydroxyproline or (2 S , 4 R ) -4-hydroxypyrrolidine-2-carboxylic acid.
Stereoisomerism
4-Hydroxypyrrolidine-2-carboxylic acid exists in four stereoisomers , in our environment only the (2 S , 4 R ) -isomer plays a role as a proteinogenic amino acid. The enantiomer of the natural (2 S , 4 R ) form is the (2 R , 4 S ) form. The (2 S , 4 S ) form and the (2 R , 4 R ) form are diastereomers of the proteinogenic (2 S , 4 R ) -4-hydroxypyrrolidine-2-carboxylic acid [synonym: (2 S , 4 R ) -4-hydroxyproline].
When hydroxyproline is mentioned in the literature and in this article - without further additives - the natural (2 S , 4 R ) -hydroxyproline [synonym: L -4-hydroxyproline] is meant. The other three isomers are of minor importance.
| Isomers of 4-hydroxypyrrolidine-2-carboxylic acid | ||||
| Surname | (2 S , 4 R ) -4-hydroxypyrrolidine-2-carboxylic acid | (2 R , 4 S ) -4-hydroxypyrrolidine-2-carboxylic acid | (2 S , 4 S ) -4-hydroxypyrrolidine-2-carboxylic acid | (2 R , 4 R ) -4-hydroxypyrrolidine-2-carboxylic acid |
| other names | L -4-hydroxyproline | trans -4-hydroxy- D -proline | ||
| Structural formula |

|

|

|
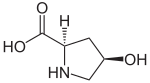
|
| CAS number | 51-35-4 | 3398-22-9 | 618-27-9 | 2584-71-6 |
| 6912-67-0 (unspec.) | ||||
| EC number | 200-091-9 | 625-221-5 | 210-542-1 | 219-963-5 |
| ? (unspec.) | ||||
| ECHA info card | 100,000,084 | 100.153.738 | 100.009.585 | 100.018.149 |
| ? (unspec.) | ||||
| PubChem | 5810 | 440074 | 440015 | 440014 |
| 825 (unspec.) | ||||
| Wikidata | Q27089020 | ? | Q27103674 | Q27101809 |
| Q411237 (unspec.) | ||||
Manufacture and extraction
biosynthesis
L -4-hydroxyproline is formed by hydroxylation in the 4-position on the pyrrolidine ring of L - proline , which is built into a polypeptide chain ( protein ) of a collagen molecule. The enzyme prolyl hydroxylase catalyzes this with the help of ascorbic acid (vitamin C). Hydroxyproline stabilizes the cohesion of the collagen triple helix in the collagen molecule via a gauche effect . The theory that hydrogen bonds emanating from hydroxyproline stabilize the triple helix has been disproved.
Commercial manufacture
The acid hydrolysis of collagen produces a protein hydrolyzate of proteinogenic α-amino acids after neutralization . L- hydroxyproline is still commercially obtained from this by ion exchange .
properties
Hydroxyproline in its pure form is mainly available as an “inner salt” or zwitterion , the formation of which can be explained by the fact that the proton of the carboxy group migrates to the lone pair of electrons on the nitrogen atom of the amino group .
The zwitterion does not migrate in the electric field because it is uncharged as a whole. Strictly speaking, this is the case at the isoelectric point (a certain pH value ), at which hydroxyproline has its lowest solubility in water. The two other stereoisomers with (2 S , 4 S ) and (2 R , 4 R ) configuration also form zwitterions.
Physiological function
Hydroxyproline is not genetically encoded directly, but arises as a post-translational modification . L- proline built into collagen is hydroxylated to L- hydroxyproline by the enzyme prolyl 4-hydroxylase and with the participation of vitamin C. Hydroxyproline is required for its mechanical properties as a structural protein . The deficiency disease scurvy results in a weak connective tissue with a reduced or absent content of hydroxyproline and hydroxylysine in the collagen.
Pathophysiology
In osteodystrophia deformans , a chronic overactivity of the osteoclasts , more hydroxyproline is released through bone breakdown. The hydroxyproline can be determined in the urine for diagnosis.
Chemistry and use
(2 S , 4 R ) - N -acetyl-4-hydroxyproline (INN: Oxaceprol ) is a drug against degenerative joint diseases. For the multi-step synthesis of an ACE inhibitor (2 S , 4 R ) -4-hydroxyproline is used as the starting material. It thermally decarboxylates by heating in tetraethylene glycol dimethyl ether in the presence of catalytic amounts of cyclohexen-3-one to form the cyclic chiral amino alcohol ( R ) -3-hydroxypyrrolidine:
Determination of the hydroxyproline content
The quantitative analysis of hydroxyproline is used to determine the proportion of connective tissue in meat products . The quality of meat products is informative, as hydroxyproline only occurs in collagen and thus mainly in connective tissue ( tendons , bones , cartilage and skin parts ); A high content of hydroxyproline is therefore an indication of an increased use of inferior raw materials . After measuring the total nitrogen according to Kjeldahl , the BEFFE value can be calculated.
The sample is digested with hydrochloric acid and hydrolyzed , whereby polypeptides are broken down into amino acids. The fat is separated and the amino acids with chloramine T oxidation . The oxidation product forms a red-colored condensation product with p - dimethylaminobenzaldehyde . This is quantitatively detected photometrically at 558 nm.
The coupling of HPLC and gas chromatography with mass spectrometry is also suitable, after appropriate sample preparation, for the reliable determination of hydroxyproline, also in addition to other amino acids in different test material.
Individual evidence
- ↑ a b c d e data sheet trans-4-Hydroxy-L-proline, 99 +% from AlfaAesar, accessed on December 26, 2019 ( PDF )(JavaScript required) .
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. 2006, ISBN 0-911910-00-X , p. 839.
- ↑ Y. Izumi et al.: Production and use of amino acids. In: Angewandte Chemie. 90 (3), 1978, pp. 187-194, doi: 10.1002 / anie.19780900307 .
- ↑ Entry on Paget's disease in Flexikon , a Wiki of the DocCheck company , accessed on September 15, 2012.
- ↑ Sabine Wallbaum, Thomas Mehler, Jürgen Martens : Decarboxylation of α-Amino Acids containing two and three Stereogenic Centers: A Simple One-Step Procedure to Prepare Two Opticall Active β-Amino Alcohols and a Bicyclic Pyrrolidine Derivative. In: Synthetic Communications. 24 (10), 1994, pp. 1381-1387, doi: 10.1080 / 00397919408011741 .
- ↑ Reinhard Mattisek, Gabriele Steiner, Markus Fischer: Food Analysis . 4th edition. Springer, Berlin 2010, ISBN 978-3-540-92205-6 .
- ↑ S. Shin, HM Jeong, SE Chung, TH Kim, SK Thapa, DY Lee, CH Song, JY Lim, SM Cho, KY Nam, WH Kang, YW Choi, BS Shin: Simultaneous analysis of acetylcarnitine, proline, hydroxyproline, Citrulline, and arginine as potential plasma biomarkers to evaluate NSAIDs-induced gastric injury by liquid chromatography-tandem mass spectrometry. In: J Pharm Biomed Anal. 165, Feb 20, 2019, pp. 101-111. PMID 30522064
- ↑ L. Konieczna, M. Pyszka, M. Okońska, M. Niedźwiecki, T. Bączek: Bioanalysis of underivatized amino acids in non-invasive exhaled breath condensate samples using liquid chromatography coupled with tandem mass spectrometry. In: J Chromatogr A. 1542, 23 Mar 2018, pp. 72–81. PMID 29477235
- ↑ M. Delport, S. Maas, SW van der Merwe, JB Laurens: Quantitation of hydroxyproline in bone by gas chromatography-mass spectrometry. In: J Chromatogr B Analyt Technol Biomed Life Sci. 804 (2), May 25, 2004, pp. 345-351. PMID 15081929


